
آخر المواضيع المضافة


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 29-12-2016
Date: 17-2-2016
Date: 17-4-2019
|
Weak bases: Ammonia
Weak bases, like weak acids, react with water to establish an equilibrium system. Ammonia is a typical weak base. It reacts with water to form the ammonium ion and the hydroxide ion:
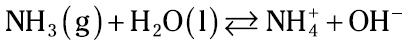
Like a weak acid, a weak base is only partially ionized. There’s a modified equilibrium constant expression for weak bases — the Kb. You use it exactly the same way you use the Ka (see Acetic acid and other weak acids” for the details), except you solve for the [OH–].



|
|
|
|
للعاملين في الليل.. حيلة صحية تجنبكم خطر هذا النوع من العمل
|
|
|
|
|
|
|
"ناسا" تحتفي برائد الفضاء السوفياتي يوري غاغارين
|
|
|
|
|
|
|
نحو شراكة وطنية متكاملة.. الأمين العام للعتبة الحسينية يبحث مع وكيل وزارة الخارجية آفاق التعاون المؤسسي
|
|
|