


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 24-11-2016
Date: 24-11-2016
Date: 27-11-2016
|
Division Coniferophyta: Conifers
CONIFERALES
The conifers are the most diverse (about 50 genera and 550 species) and familiar of the gymnosperms (Fig. 1; Table ). They are all trees of moderate to gigantic size; the giant redwoods of California (Sequoiadendron giganteum) reach 90 m in height and 10 m in diameter. Conifers are never vines, herbs, or annuals, and they never have bulbs or rhizomes.
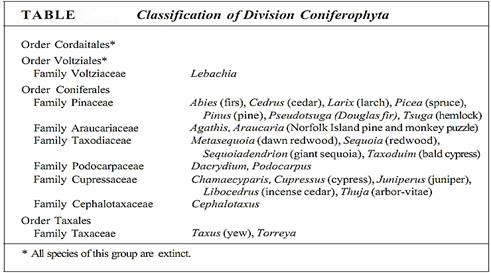

FIGURE 1:Division Coniferophyta is large and diverse, containing many familiar plants, (a) Ponderosa pine (pinus ponderosa) (Runk/Schoenberger from Grant Heilman). (b) American larch (Larix laricina), which is deciduous, losing its leaves in autumn. (R Stottlemyer, Michigan Technological University/Biological Photo Service) (c) Big tree redwood (Sequoiadendrongiganteum). (J. N. A. Lott, McMaster University/Biological Photo Service) (d) Garden juniper (Juniperus chinensis var. procumbens), which grows as a flat ground cover. (B. L. Runk from Grant Heilman).
The leaves of conifers are always simple, being either needles or scales. Leaves of most conifers are perennial, persisting for many years (Fig. 2); leaves of Agathis and Araucaria remain even on very old trunks (Fig. 3).

FIGURE 2:Conifer leaves are never compound. They are always simple and leathery, needle-like in some, scalelike in others. (a) In eastern or Canadian hemlock (Tsuga canadensis), each leaf has two white bands where stomata are located. (Grant Heilman) (b) Scalelike leaves of Chamaecyparis lawsoniana. (William £. Ferguson) (c) Pines have two types of leaves—small, papery brown scales on the long shoots (visible on the branch) and the long needles borne by the short shoots located in the axils of the papery scale leaves. The long needles were produced last year. White pine (Pinus strobus). (E. R. Degginger).
The venation of conifer leaves is often simple, with just one or two long veins running down the center of a needle-shaped leaf, or several parallel veins in scale-shaped leaves. Unlike the leaf veins of flowering plants, those of conifers have an endodermis (Fig. 4). Furthermore, in addition to the ordinary vascular tissues of the leaf vein, there is also a tissue called transfusion tissue, consisting of transfusion parenchyma cells and transfusion tracheids. The latter are more or less cuboidal and have prominent circular bordered pits. Transfusion parenchyma is intermixed with the tracheids, the two cell types forming a complex three-dimensional pattern that apparently facilitates the transfer of materials between the ordinary vascular tissues and the mesophyll tissue outside the endodermis.
Just as in their progymnosperm ancestor Archaeopteris, the wood of modern conifers lacks vessels and their phloem lacks sieve tubes. Tracheids are so narrow that only one or two rows of circular bordered pits can occur on their radial walls . All conifers have pollen cones and seed cones, most of which are woody, but in Juniperus and Podocarpus, seed cones superficially resemble the fruits of flowering plants. The conifers are still a very successful group, forming extensive forests covering over 17 million km2; in many of these forests, flowering plants exist only as herbs, shrubs, or small trees growing in the conifers' shade.

FIGURE 3:In almost all species of conifers, each leaf lives for several years, or as in the case of this Araucaria, for many years, persisting even as the trunk becomes massive.
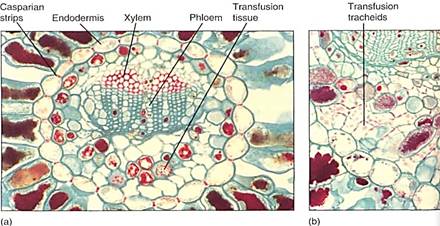
FIGURE 4: (a) The vascular bundle of this Douglas fir leaf has an endodermis, xylem, phloem, and transfusion tissue (X 80). (b) High magnification of a pine leaf bundle showing secondary phloem and transfusion tracheids. Casparian strips are visible in the endodermis walls (X 130).
The pines are good representatives for closer examination. The trees are monopodial, with one main trunk bearing many branches; the wood is composed exclusively of tracheids, but annual rings, spring wood, and summer wood are all visible because large-diameter tracheids are produced in the spring, followed by narrow-diameter tracheids in the summer (Fig. 5). Rays are thin and tall and contain both ray parenchyma and ray tracheids. Resin canals, which produce the thick, sticky pitch, run vertically among the tracheids and horizontally in the rays. The wood has almost no axial parenchyma. Phloem contains sieve cells and albuminous cells and also has tall, narrow rays (Fig. 6). A oak cambium produces a thick, tough bark that provides excellent protection even from forest fires.
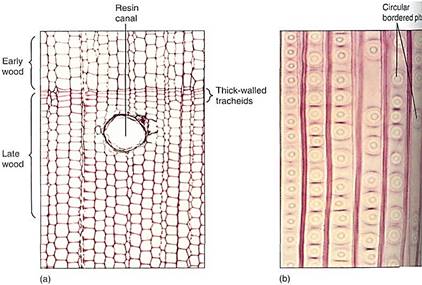
FIGURE 5: (a) The wood of pine, like that of all conifers, lacks vessels; it consists almost exclusively of tracheids. Growth rings are visible because late wood contains narrow, thick-walled tracheids, whereas early wood contains wide, thin-walled tracheids (transverse section, X 30). (b) Pine tracheids are narrow and their circular bordered pits are wide, so only one row of pits fits on a given wall, unlike the dozens of small pits that cover each wall of an angiosperm vessel (X 80). Note how similar this wood is to that of progymnosperms .
FIGURE 6:Phloem of conifers contains sieve cells, not sieve tube members; sieve cells are long and narrow with sieve areas over much of their surface, but they never have horizontal cross walls (sieve plates) with enlarged sieve pores. The sieve areas in this pine are particularly abundant and easy to see (X 25).
Pines, like several other conifers, have two types of shoot, each with a characteristic type of leaf. Tiny papery leaves occur on long shoots and in their axils are short shoots that produce the familiar long needle leaves (see Figs. 2c and 7). The leaves have many xeromorphic characters: thick cuticle, sunken stomata, cylindrical shape.

FIGURE 7:In Cedrus atlantica, the nature of the short shoot is more obvious because it forms more needles each year and so slowly grows into a visible shoot.
Like all conifers, pines have both pollen cones and seed cones. Pollen cones are simple cones; that is, they consist of a single short unbranched axis that bears microsporophylls (Figs. 8 and 9). Microspore mother cells undergo meiosis and form microspores; then each of these develops endosporially into a small gametophyte with four cells, one of which is a generative cell as in flowering plants (Figs.10 and11). The gametophytes are shed from the tree as pollen and carried by wind; a small percentage land in seed cones, but the great majority land elsewhere and die.

FIGURE 8: (a) Pollen cones typically occur in clusters near the ends of branches. As the microsporangia dehisce, pollen is liberated to the wind and blown away for distribution. Such a method of gene transfer is inefficient because so few pollen grains land on seed cones; it is successful primarily because conifers grow as dense forests, where each conifer is surrounded by hundreds of potential partners. (b) Pollen cones are simple cones; they have one single stem axis and bear microsporophylls. These are not microsporangia but rather leaf-like structures that bear microsporangia (X 10). (Robert and Linda Mitchell).

FIGURE 9 :In genera such as Araucaria, each microsporophyll is obviously one sporophyll carrying two sporangia. In Cupressus and Pinus, the sporophyll has been so simplified that it looks like a lobed sporangium rather than a sporophyll with several sporangia.
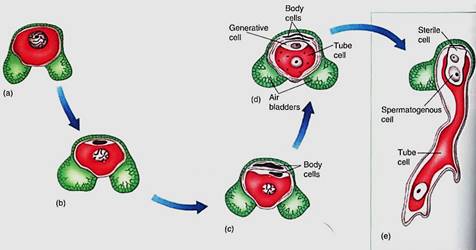
FIGURE 10:The microspore (pollen) of pine has one cell and two large air bladders that increase its buoyancy in air. The microspore develops into a small gametophyte in a process similar to that in pollen of flowering plants, except that a few more body cells are formed. First, two mitotic divisions produce two small body cells that degenerate and a large cell that divides, resulting in a generative cell and another body cell, called the tube cell. As in angiosperms, the body cell becomes He pollen tube and the generative cell divides into two nonmotile sperm cells.
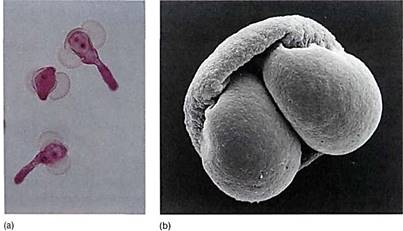
FIGURE 11: (a) Young pollen grains in which one large body cell and the generative cell are visible (X 200). (E. R. Degginger/Earth Scenes) (b) The two air bladders of pine pollen are easily visible in this scanning electron micrograph. The addition of the hollow bladders adds virtually no weight to the pollen but increases its volume so that the pollen's density (weight per volume) is decreased and it does not sink so quickly in air (X 3000). (R E. Litchfield/Science Source/Photo Researchers, Inc.).
Seed cones are more complex than pollen cones: They are compound cones, each consisting of a shoot with axillary buds. The short axis bears leaves called bracts rather than sporophylls (Fig. 12). Each bract has an axillary bud that bears the megasporophylls. In some fossil conifers the individual structures can still be seen, but in all modern conifers, extensive fusion has occurred: The axillary bud is microscopic and its megasporophylls are fused laterally, forming an ovuliferous scale (Figs. 13 and 14). In larch, fir, and Douglas fir (Pseudotsuga) the ovuliferous scale is still distinct and can be seen, but in most conifers it is fused to the bract.
Inside each megasporangium, a single large megaspore mother cell undergoes meiosis, with three of the resulting cells degenerating and only one surviving as the megaspore (Fig. 15). The megasporangium does not dehisce; the megaspore is retained inside and glows into a large coenocytic megagametophyte by free nuclear divisions and may have as many as 7200 nuclei.

FIGURE 12: (a) The long three-pointed bracts of this Douglas fir (Pseudotsuga) cone are the true leaves of the cone axis. Each one contains an axillary bud whose leaves have fused together side by side into the flat, shieldlike ovuliferous scale just behind each bract. These fused leaves that constitute the ovuliferous scale are actually megasporophylls. (b) If an ovuliferous scale were pulled from the cone and turned over, the two ovules would be visible. The scale obviously does not look like a set of leaves fused together, but look at Figures 13.
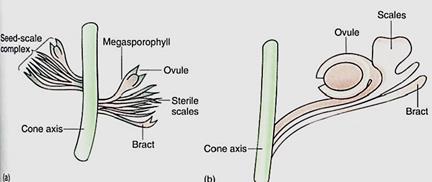
FIGURE 13: Possible steps in the evolution of seed cones: (a) In Lebachia, an early conifer, the bract is visible and the nature of the fertile axillary bud is obvious. Only slight fusion of megasporophylls had occurred. (b) In the Triassic Voltzia, the modern condition was essentially complete, with sporophylls fused together.

FIGURE 14: (a) In the seed cones of pine, all parts are fused together; even the bract is fused to the ovuliferous scale. When morphologists first began working on pine cones without knowing about fossil structures, they were completely baffled and could not explain such a complex structure. (William E. Ferguson) (b) Colorado blue spruce (Piceapungens). (Runk/Schoenberger from Grant Heilman) (c) Rock "cedar" (Juniperus ashei). (d) Atlantic cedar (Cedrus atlantica). (R.J. Erwin/ Photo Researchers)
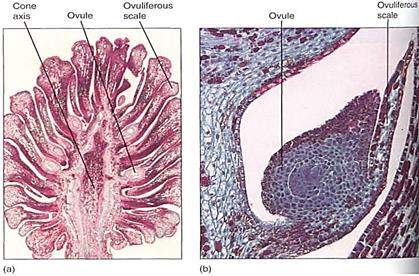
FIGURE 15:This section of a pine cone was made just as the megaspore mother cells were about to begin meiosis. Each ovuliferous scale carries two ovules, each of which contains an integument and a nucellus (the actual megasporangium). As in flowering plants, each nucellus usually has only one megasporocyte (megaspore mother cell) and produces only one surviving megaspore. (a) X 2. (Bruce Iverson) (b) X 25. (Robert and Linda Mitchell)
into a large coenocytic megagametophyte by free nuclear divisions and may have as many as 7200 nuclei. Development can take as long as a year, but finally walls form, converting the coenocyte into a cellular megagametophyte. Two or three archegonia form as sets of cells, each surrounding a large egg (Fig. 16). Conifer eggs are gigantic cells loaded with carbohydrate and protein. The egg nucleus, although haploid, is swollen to a volume much larger than that typical for entire cells. It is probably filled with DNA synthetases and RNA polymerases, ready for extremely rapid activity once karyogamy occurs.
Unlike pollination in flowering plants, conifer pollen arrives before the egg is mature, and more than a year may pass between pollination and fertilization. The pollen germinates, and a massive pollen tube slowly digests its way toward the megagametophyte as the egg forms. Because the megasporangium does not open, a passageway digested by a pollen tube is necessary. The two or three eggs in one megagametophyte can all be fertilized, but only one zygote develops into an embryo; the other one or two die. A zygote does not immediately form an embryo in conifers; instead, some of the first cells elongate as a suspensor that pushes the other cells deep into the megagametophyte (Fig. 17). These other cells, called the proembryo, develop into the embryo. No double fertilization occurs; rather, the female gametophyte continues to grow and acts as a nutritive tissue similar to endosperm. The mature embryo has the same organization as an angiosperm embryo (radicle, hypocotyl, epicotyl, and cotyledons) but always has many cotyledons, not just one or two (Fig. 17d). The seed also resembles that of a flowering plant, but it is borne in a cone, not a fruit. In two conifers the cones become fleshy, fruitlike, and brightly colored— red in Podocarpus, blue in Juniperus (see Fig. 14).
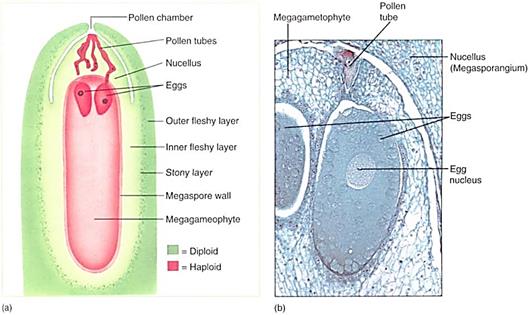
FIGURE 16:Ovules of pine and other conifers are much larger than those of flowering plants. This cellular megagametophyte is not as large as a moss or liverwort gametophyte, but it is much more plantlike than the megagametophytes of angiosperms . Above the two eggs is the thick, indehiscent megasporangium (nucellus). Sperms cannot swim to the archegonia and eggs; they must be carried by a pollen tube that digests its way through the megasporangium. Between the megasporangium and the integuments is the pollen chamber (X 20). (Photograph: James W. Richardson/CBR Images).
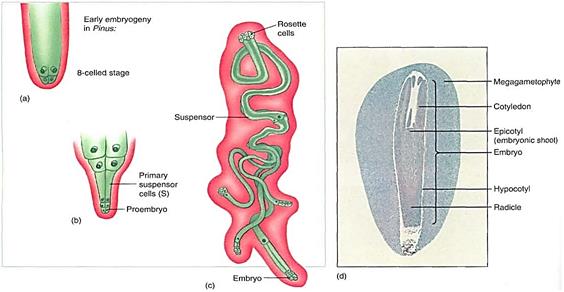
FIGURE 17: (a) Just as in angiosperms , the zygote of conifers does not immediately form an embryo; instead it produces a suspensor that thrusts the proembryo into the megagametophyte (b). (c) In conifers the suspensor can divide and form multiple embryos from each zygote; they do not produce identical twins or quadruplets, however, because only one embryo survives. Also, even though each megagametophyte produces several eggs, only one embryo survives in each ovule (with rare exceptions). (d) A conifer seed looks remarkably like the seed of a flowering plant. It has a seed coat (removed here) derived from the integument, an embryo with several cotyledons, and a nutritive tissue that is actually the megagametophyte, not endosperm. After fertilization, the parental sporophyte transports large amounts of nutrients, supplying everything needed for the embryos, which grow completely heterotrophically. It also fills all cells of the megagametophyte with carbohydrates, proteins, and mineral nutrients; this acts just like the endosperm of flowering plants and is often called endosperm (X 2.5).
ORIGIN AND EVOLUTION OF CONIFERS
As early as 360 million years ago, progymnosperms had a vascular cambium that produced secondary xylem and phloem similar to that in modern conifers. In fact, the only wood characters absent from Archaeopteris but present in some modern conifers are resin canals and axial wood parenchyma. The earliest resin canals are found in Lower Cretaceous fossilized wood called Pityoxylon, the form genus for wood with characters of the extant genera Larix, Picea, Pinus, and Pseudotsuga. Axial wood parenchyma may have arisen independently several times. It is found in fossil wood similar to that of Taxodiaceae and Cupressaceae as well as in fossil wood similar to that of Podocarpus and Dacrydium.
Starting in strata about 300 million years old (late Carboniferous), fossils are fund that are considered to be basically true gymnosperms. They are assigned to the groups Cordaitales and Voltziales . Cordaitales were small to large trees with gymnosperm wood (Fig. 18). Their leaves (form genus Cordaites) were strap-shaped and up to 1 m long and 1.5 cm wide. Leaf veins appeared to be parallel because the leaves were so long; the veins actually dichotomized and resembled those of Archaeopteris macilenta . Voltziales resembled the extant plants known as Norfolk Island pines (Araucaria excelsa); they had tall trunks, branches in whorls, and needle-shaped leaves that could have been derived from telomes of Archaeopteris fissilis.
The most significant events in the transition of progymnosperms to gymnosperms was the evolutionary modification of reproductive, structures. The progymnosperm Archaeoptens was heterosporous, but its sporangia were relatively exposed and unprotected; if fossils of the form genus Archaeosperma (seeds) were part of Archaeopteris, as is suspected, there was a cupule and an integument around the megasporangium. Reproductively, Cordaitales and Voltziales had fewer relictual features than Archaeopteris and Archaeosperma. Megasporangiate structures of Cordaitales were somewhat similar to modern seed cones of conifers in that they had a primary shoot with sterile bracts; in the bract axils were secondary shoots with fertile and sterile leaves. In some members of Voltziales the secondary shoots had become planar and bilaterally symmetrical . In Pseudovoltzia ,the sterile and fertile leaves of the secondary shoots had become fused, like an ovuliferous scale in modern conifers. By the Cretaceous, there was a cone, classified in the form genus Compsostrobus, that was almost identical to a pine cone except that its bracts were longer and it was less compact.
In many features, Voltziales were almost identical to modern conifers, and they existed into the Jurassic Period, long after the first modern conifers appeared. Voltziales were so similar to Coniferales and the final transition was so gradual that a distinct division between the two groups is difficult to make.

FIGURE 18:Cordaites was a small tree with long, strap-shaped leaves.
TAXALES
The order Taxales contains only one family, Taxaceae, with five genera, Taxus (yew) and Torreya being the most familiar . Its members strongly resemble species of Coniferales except in some aspects of their reproduction: Rather than having seed cones like the conifers, the taxads bear their seeds terminally on short lateral shoots (Fig. 19). They have no cone and after fertilization, as the seed develops, a bright red, fleshy, sweet envelope, the aril, grows around the seed. Microsporangia occur in groups of up to nine on flat microsporophylls.
Members of the Taxales are separated from the Coniferales on the basis of their arillate seed and lack of a typical seed cone. Much of our understanding of gymnosperms is due directly to the extensive and careful work of R. Florin; he concluded that the megasporangiate structure of taxads could not be traced to the Cordaitales or Voltziales but rather must have derived from sporangia similar to those of Rhynia. If this is correct, the Taxales are quite distinct phylogenetically from Coniferales. This would mean that megaphylls, roots, a vascular cambium, and secondary growth evolved independently in the taxad line of evolution. But the structure of taxads is so similar to that of conifers that this conclusion is difficult to accept. More recently, it has been suggested that the taxad arillate seed raid have evolved from a Lebachia-type of reproductive shoot by simple modifications. If so, the Taxales are closely related to the conifers.

FIGURE 19: (a) Taxus has individual seeds, each surrounded by a red, fleshy aril. In the young seeds (grey) the aril is only partly expanded. Taxus cuspidata. (E. R Degginger) (b) Taxus pollen "cones" are not as tighty clustered as those of conifers but otherwise are not very different. (George Lour/Visuals Unlimited).



|
|
|
|
التوتر والسرطان.. علماء يحذرون من "صلة خطيرة"
|
|
|
|
|
|
|
مرآة السيارة: مدى دقة عكسها للصورة الصحيحة
|
|
|
|
|
|
|
نحو شراكة وطنية متكاملة.. الأمين العام للعتبة الحسينية يبحث مع وكيل وزارة الخارجية آفاق التعاون المؤسسي
|
|
|