


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 24-10-2016
Date: 7-11-2016
Date: 31-10-2016
|
Communication Within The Plant
PERCEPTION AND TRANSDUCTION
Many, possibly most, responses occur in tissues or organs different from those that sense the stimuli. The site of perception is not the site of response, so a form of communication must exist. In plants, most sites of perception and response are not specialized for those functions but seem to be rather ordinary cells. Day length is probably perceived by all living leaf cells; no specialized region of cells has been discovered. Low temperatures for vernalization appear to be detected by buds, which do not contain a particular group of cells specialized just for temperature perception. In root caps, certain cells called statocytes do have large starch granules, statoliths, that sink in response to gravity; they are too dense to float in cytoplasm and always settle to the bottom of the cell, thereby distinguishing "down" from "up" (Fig. 1). This is our best example of a set of specialized perceptive cells. The trigger hairs on Venus' flytrap leaves are also a discrete perceptive mechanism, but it is not known which cells within the hairs are responsible.
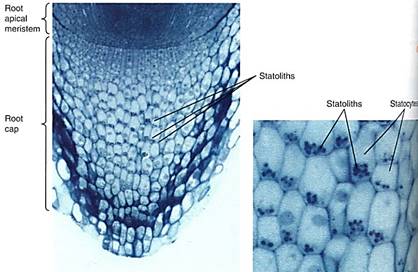
FIGURE 1:Cells located centrally in this root cap are statocytes, and their starch grains are statoliths. Regardless of vertical or horizontal position, the statoliths are located at the gravitationally lower side. It is necessary to distinguish between the gravitational and the morphological bottom in gravity-sensing systems. (Courtesy of R. Moore and E. McClellan, Baylor University)
The site of perception is tentatively assumed to be the site of transduction, where the stimulus is converted into a form that can be transmitted and can trigger a reaction at a response site . Transduction is still a complete mystery in almost all plant responses; we do not know how changes in temperature, light, weight, or humidity are converted into chemical signals.
Two factors are important in perception and transduction: presentation time and threshold. Presentation time is the length of time the stimulus must be present lor the perceptive cells to react and complete transduction. Presentation time for root gravitropism is easy to understand: A root must lie on its side long enough for statoliths to sink to the new bottom of the cell. If the root is returned to vertical before they can settle, no perception occurs. In many tropic responses, only a brief touch or unilateral lighting is to cause curvature; presentation times are often only a few seconds.
Once the stimulus has acted long enough to fulfill the presentation time, a response occurs even if the stimulus is removed. For example, the vernalization of many biennial plants has a presentation time of only one or a few days; after this, the plants still flower at the proper time even if kept in warm, non-vernalizing conditions. Tendrils of peas do not bend thigmotropically in the dark, but if they are rubbed for several seconds—their presentation time—in the dark, they bend when placed in light even though they are no longer being touched.
Threshold refers to the level of stimulus that must be present during the presentation time to cause perception and transduction. In phototropism, plants are extremely sensitive to very dim unilateral light if they are in an otherwise dark environment; the threshold for curvature is low. However, in bright conditions the threshold is higher, and the unilateral light must be much stronger to trigger curvature. In Venus' flytraps, the threshold for stimulating leaf closure is moderate: The trigger hairs must be firmly bent. This is advantageous in preventing wind or rain from triggering trap closure; the moderate threshold almost guarantees that the trap contains an insect every time it closes (Fig. 2).

FIGURE 2: Threshold must be appropriate to the amount of change a stimulus can cause; Venus' flytrap hairs with an extremely low (sensitive) threshold would be capable of detecting and catching every insect, but wind and rain would cause so many useless closings that the leaf would be inefficient because it would miss insects whenever it was closed unnecessarily. With a medium threshold, it captures more insects because it is open and ready much of the time. Small insects may escape because they cannot bend the trigger hairs enough to meet the threshold. If the threshold were too high, no insects would be caught because none could bend the hairs. The trap would close only when larger animals brushed against it, but these animals are too big to be enclosed in the trap, so all closures would be unproductive. We can hypothesize that natural selection results in a threshold appropriate for the most abundant size of insects.
Related to threshold is the level of response relative to the level of stimulation; the alternatives are all-or-none responses and dosage-dependent responses. In an all-or-none response, once the threshold and presentation time requirements are met, the stimulus is no longer important; the response is now completely internal. Individuals respond identically whether they received strong or weak stimuli, regardless of whether the stimulus was brief or long lasting. For example, many species are induced to flower by environmental conditions; once the minimal threshold and presentation time requirements are met, the plants flower fully, limited only by their general health, vigor, and nutrient reserves. Until they receive the proper stimulus, they produce no flowers; their flowering is all or none. Examples are poinsettia, chrysanthemum, Hybiscus syriacus, and oats.
In dosage-dependent responses, the amount or duration of the stimulus affects the amount or duration of the response. In species of this type, individuals that receive only minimum stimulation flower poorly, even if the plant is quite healthy. Those that receive longer or stronger stimulation produce more flowers. Examples are turnip, marijuana (Cannabis sativa), and some varieties of cotton and potato.
CHEMICAL MESSENGERS
Almost all plant communication is by a slow mechanism: transport of hormones through the plant. Hormones are organic chemicals that are produced in one part of a plant and fan transported to other parts, where they initiate a response. A critical aspect is that hormones act at very low concentrations. Hormones are synthesized or stored in regions of transduction and are released for transport through either phloem or mesophyll and cortical cells when the appropriate stimulus occurs. At the site of response, hormones bind to receptor molecules, probably located in the plasma membrane or some other membrane, and thereby trigger a response. Hormones appear to be released into general circulation and are not carried specifically to the target. Many regions that are not target regions are exposed to the hormone but do not respond because they do not have the proper receptor molecules. In some instances, a plant hormone acts directly on the cells that produce it.
At one time, plant hormones were believed to carry in their structure much of the information necessary for the response. We now know that plant hormones are quite simple in structure. The receptor cell and its nucleus contain almost all the information necessary for proper response, and hormones serve only to activate the response. An analogy is a computer, its programs, and commands. The computer is capable of carrying out numerous functions and processes but only if properly controlled; the same is true of cells, tissues, organs, and whole plants. Computer programs contain the information needed to run the computer, just as the nuclear, plastid, and mitochondrial genes contain the information needed to run cells. Both computer programs and genomes contain numerous sets of information. On a computer, commands such as PRINT, STORE, COPY, and EDIT select subroutines or programs. Hormones are thought to act as commands that activate programs within the target cells (Fig. 3a and b). In the early 1980s it was finally proven that the activation of genes and the initiation of the production of new proteins needed for the new type of metabolism that is part of the response is one of the effects of hormone action.
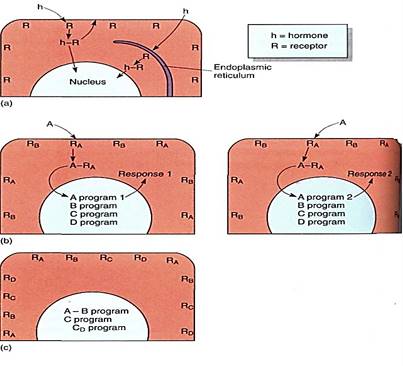
FIGURE 3:A cell responds to a hormone only if it has receptors for that hormone. (a) Evidence suggests that some receptors (R) are in the plasma membrane and others are in membranes such as the endoplasmic reticulum. Once bound, the enzyme-receptor complex (h-R) may cause a metabolic change immediately, or the complex may migrate to another site, such as the nucleus. (b) In many responses, some nuclear genes are activated and others are repressed. Cells may have receptors for several hormones (RA and RB); if hormone A is present, it binds and activates (or represses) program A. Other programs are unchanged. One of the results of program A might be to withdraw the receptors of either A or B from the membrane or to add receptors for C or D, thus changing the sensitivity and type of response possible. A second cell (the cell on the right) may have a different program (A program 2) activated by hormone A; the response is cell specific, not just hormone specific. (c) The effects of a hormone are often quite different when the hormone is applied alone, or with or after a second hormone. In this hypothetical cell, development occurs only if hormones A and B are applied simultaneously or if hormone D is applied after hormone C
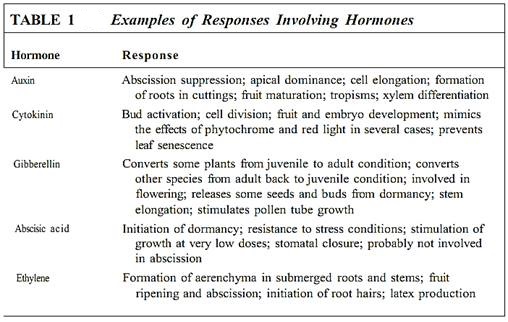
In higher animals, because so many systems and responses must be activated, many distinct hormones are necessary. Because plants are much simpler, their responses can be controlled by fewer hormones; even so, the handful of known plant hormones seems inadequate. It is likely that many plant hormones are still unknown to us. Also, many responses are activated not by one hormone but by a combination or a sequence of several hormones (Fig. 14.12c), or a particular hormone elicits different responses when present at different concentrations. The following are examples of the most well-studied hormones.
Auxins. The first plant hormone discovered was auxin. In 1926 it was identified as the chemical messenger involved in positive phototropism in oat seedlings. Identifying it chemically at that time was impossible because it is present in such low concentrations. Experiments had to be performed by allowing auxin to diffuse out of a seedling leaf tip into a small block of agar, which was then used as if it were a small dose of auxin . The auxin was later identified as being indole-acetic acid (IAA), which could be synthesized artificially and applied to plants under various conditions to find other responses that IAA might either mediate or inhibit (Fig. 4). The search was successful—dozens of responses were found (Table 1). Many compounds chemically related to IAA were also found to be as effective or even more so. It was hypothesized that IAA was only one of many natural auxins, each with its own effect and role, but further findings were not consistent with that hypothesis. Analysis of IAA metabolism showed that the compounds were converted to IAA by the plant's enzymes. At present some evidence suggests that phenylacetic acid and chlorinated IAA act directly as natural auxins without being converted to IAA.
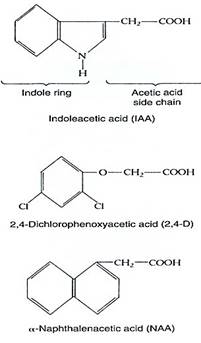
FIGURE 4 :Indoleacetic acid is the natural hormone auxin; the others are synthetic so they are called plant growth substances.
Because we are not certain how many natural auxins there are, any compound that imitates IAA in controlling aspects of plant differentiation is called an auxin. For example, any chemical that gives a response similar to IAA in oat seedling phototropism is consider to be an auxin. Experiments are often performed by applying IAA to a plant and examining the responses. When such extra IAA causes a response, we suspect that in normal development, auxin is involved. But we cannot be certain that it is actually IAA that participates in natural conditions. It may be that another, undiscovered auxin is normally involved and in the experiment we have overwhelmed it with IAA. Because of this uncertainty, the word "auxin" is often used when we have not yet identified the chemical messenger involved.
Many synthetic compounds mimic the effect of auxin or the other hormones; for clarity, only natural products are called hormones. The term "plant growth substance” is used for any hormone-like compound, natural or artificial (Fig. 4f ). Naphthaleneacetic ail, an artificial compound, produces effects in plants that are for the most part indistinguishable from those of IAA. 2,4-Dichlorophenoxyacetic acid (2,4-D) is auxin-like but so powerful that it disrupts most normal growth and development even in low concentrations, making it valuable as an herbicide.
Indoleacetic acid is related to the amino acid tryptophan; so far, at least four separate metabolic pathways are known that convert tryptophan to IAA. Some plants, corn for example, synthesize IAA by different pathways at different stages of development. The significance of this is not clear, but each pathway has its own characteristic set of controls] The most active centers of auxin synthesis are shoot apical meristems, young leaves, and fruits. It is present in root tips, but is believed to be transported there from the shoot rather than being synthesized there.
The concentration of substances as powerful as hormones can be controlled not only by synthesis but also by destruction and by conversion to an inert storage form. Two pathways for IAA destruction are known: removal of the side group and oxidation of the five-member ring. IAA is converted to an inactive form by conjugating (attaching) it to various compounds (Table 2). In the conjugated form, IAA is safe from destruction, it can be stored indefinitely in seeds, and it can be transported from cotyledons to the epicotyl during germination. Conjugation allows rapid regulation of the level of free IAA. Up to 80% of the IAA in oat seeds is conjugated, but this can be de-conjugated, releasing free IAA during germination more quickly than synthesis could.
In addition to hormone transport by phloem, a second mechanism exists for auxin only: polar transport. In shoots and leaves, auxin moves basipetally—from the apex to the base of the plant, and in roots it moves acropetally toward the root apex. Movement is about 11 mm/hr regardless of whether the tissue is in a vertical, horizontal, or upside-clown orientation. By means of the polar transport system, auxin movement through the sinks and sources for carbohydrates and minerals.
Cytokinins. Cytokinins were named for the fact that their addition to a tissue culture medium containing auxin and sugar stimulates cell division—cytokinesis (Fig. 5). The first one discovered, kinetin, is an artificial cytokinin, but two natural ones, zeatin and isopentenyl adenine, have been found, and more are suspected to exist (Fig. 6). Cytokinins are purines, related to adenine; extensive testing of adenine analogs has been done to determine which aspects of its chemical structure are critical to the molecule's ability to act as a cytokinin. The most active compounds have a side group containing four to six carbon almost attached to C6. If this side group is longer or if complex groups occur at other areas, the molecule does not act like a cytokinin, apparently lacking the proper shape and charge to bind with the cytokinin receptor molecule.
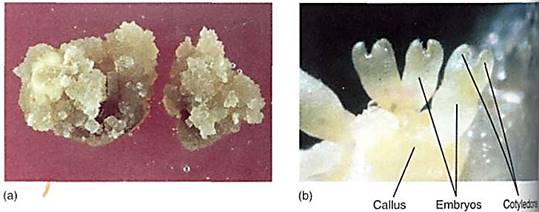
FIGURE 5 :Cells of most dicots, such as this tobacco, can be grown in culture if provided with auxin, cytokinin, some vitamins, minerals, and sugar. The ratio of auxin to cytokinin is important, as are the absolute concentrations. (a) At one ratio, the cells proliferate as a callus composed of parenchyma. (Biological Photo Service) (b) At another ratio of auxin to cytokinin, buds form in the callus and then grow into shoots. (Courtesy of Dennis Gray, University of Florida)
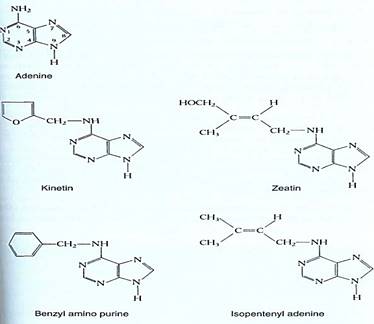
FIGURE 6 : Both natural and artificial cytokinins are related chemically to adenine. The size and chemical nature of the group on C6 is critical.
Several enzymes carry out the destruction or conjugation of cytokinins, but their significance is not obvious. The enzymes work rapidly on cytokinins added to tissues experimentally, but natural, endogenous cytokinins often appear to be immune to them. Corn kernels have high levels of both cytokinin oxidase and zeatin. Perhaps the two are compartmentalized in separate organelles within cells.
Like auxin, cytokinins are involved in dozens of responses in all parts of the plant . One important response is root-shoot coordination. As roots begin to grow actively in the spring, they produce large amounts of cytokinins that are transported to the shoot, where they cause the dormant buds to become active and expand. The richest concentrations of cytokinins generally occur in endosperm and are apparently involved in controlling the development and morphogenesis of the embryo and seed.
Gibberellins. At least 62 gibberellins are known, and rather than being named, they are just numbered: GA1, GA2, . . . GA62 (Fig. 7). The most abundant and perhaps most important, GA3, has the name gibberellic acid. Gibberellins have diverse functions but a unifying structure, the gibberellane ring system. This class of hormones is thus defined by structure: A compound cannot be a gibberellin if it does not have the gibberellane ring system.
Gibberellin metabolism is complex. Only a few gibberellins are known to be active as hormones; others are precursors or intermediates in transforming one active form into another. Relative concentrations of the various gibberellins change in response to environmental signals. When spinach is exposed to long days (summer conditions), the level of GA19 undergoes a fivefold decrease, GA20 and GA29 increase drastically, but GA17 and GA44 do not change. Gibberellin metabolism appears to occur in all parts of the plant, but seeds, roots, and leaves are especially important. Like all other hormones, gibberellins are involved in numerous responses (Fig. 8).
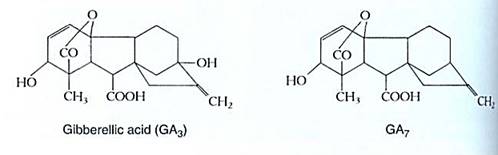
FIGURE 7 :All gibberellins are based on gibberellane; the most common forms are GA3 and GA7. Not all of the 62 gibberellins occur in plants; at least 15 have been found only in fungi.

FIGURE 8: (a) Many biennial plants grow as a rosette in their first year, then elongate rapidly (bolt) in their second year, producing a tall shoot that bears numerous flowers. The bolting is controlled by gibberellic acid; if it is absent, the plants remain short. (Robert E. Lyons) (b) In crop plants, having tall stalks often makes harvesting difficult, and it is a waste of energy in fields where there is no competition for light. Plant breeders often artificially select mutant plants that are dwarfed or short. Many of these dwarf forms either lack the particular gibberellin needed for stem elongation or are unresponsive to it, perhaps because they lack the proper receptors. (Courtesy of B. O. Phinney, University of California, Los Angeles)
Abscisic Acid. This class apparently contains the single compound abscisic acid (ABA) (Fig. 9). As its name suggests, it was thought to play a role in the abscission of fruit, leaves, and flowers, but at present we do not know whether that is true of many species or only sycamore, the species in which it was discovered. ABA is widely regarded as a growth inhibitor, possibly involved in inducing dormancy in buds and seeds. However, dormancy is not just cessation of growth, but a complicated set of changes that prepare the plant or seed for adverse conditions. It is incorrect to equate induction of dormancy with mere growth inhibition.
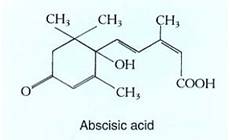
FIGURE 9: Abscisic acid is transported rapidly between cells and through phloem, so its presence in a tissue is not proof that it was produced there. ABA can be synthesized from mevalonic acid in roots, stems, leaves, fruits, and seeds of various species.
The role of ABA in all types of stress resistance is receiving great attention; heating of leaves, waterlogging of roots, chilling, and high salinity have all been found to cause sudden increases in ABA. If healthy plants are pretreated with ABA, they become much more resistant to stressful conditions. When plants begin to wilt, the concentration of ABA in leaf cells increases dramatically from about 20 µg/kg fresh weight to 500 µg/kg, and guard cells close stomatal pores. Wilt-induced production of ABA overrides all other stomata controls; other mechanisms that normally cause stomata to open are ineffective when ABA is present. The stimulus for ABA production appears to be loss of turgor. Large amount of ABA are exported from wilted leaves to the rest of the plant, moving through phloem
ABA can be removed by being converted to phaseic acid, which has no known hormone activity. ABA can be conjugated to glucose, but the conjugation may not be a useful, recoverable storage form. As plants wilt, the concentration of free ABA rises suddenly but that of conjugated ABA does not drop. Beets (Beta vulgaris) labeled with radioactive ABA conjugate did not release any of the radioactive ABA when allowed to wilt.
Elytra Ethylene is the only gaseous plant hormone, and it has the simplest structure (Figure 10). Its most commonly studied effects occur during fruit development. Fruits such as apple, avocado, banana, mango, and tomato are climacteric fruits: They ripen slowly as they mature, but in the final stages, numerous developmental changes occur rapidly. Starches are converted to sugars, cell walls break down and soften, flavors and aromas develop, and color changes. Ethylene stimulates these changes, but at first so little ethylene is present that the changes occur slowly. However, one effect of ethylene in these fruits is the production of more ethylene, which constitutes a positive feedback system: The concentration increases exponentially and rapidly. The sudden burst of ethylene and the rapid completion of maturation of the fruit are known as a climacteric; at this time ethylene production can be as high as 320 nl/g/hr (one nl = one nanoliter = one billionth of a liter). In nonclimacteric fruits, such as cherry, lemon, and orange, ethylene does rot stimulate its own production, so ethylene levels remain stable and no sudden change occurs just before maturity.
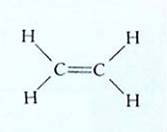
FIGURE 10 :Ethylene is a simple, small molecule. Many of its effects are blocked by carbon dioxide, whose size and shape are similar enough to those of ethylene that it can bind to ethylene's receptor and block normal response.
Because ethylene controls the ripening of most of our important food fruits, it is important commercially. It is used in harvesting cherries, cotton, and walnuts by causing their uniform abscission; it also synchronizes flowering and fruiting in pineapple, making harvesting easier. Ethylene can be drawn out of unripe fruits by storing or transporting them in a partial vacuum. When they reach market, air pressure is returned to normal, ethylene accumulates, and ripening occurs. Fruits may also be treated with 2-chloro- ethylphosphonic acid (commercial trade name Ethrel), which breaks down and releases ethylene.
Being a gas, ethylene moves rapidly through tissues by diffusion rather than by specific transport mechanisms. In many cases, it acts as a final effector for auxin. Arrival of auxin at a target site often causes that site to produce ethylene, which can diffuse rapidly and trigger responses in the adjacent area more quickly than the auxin itself could. Ethylene can be broken down to either carbon dioxide or ethylene oxide, and when radioactively labeled ethylene is supplied experimentally to bean tissues, it becomes bound. It is not known whether binding is a form of storage or if the hormone is attaching to its receptor.



|
|
|
|
علامات بسيطة في جسدك قد تنذر بمرض "قاتل"
|
|
|
|
|
|
|
أول صور ثلاثية الأبعاد للغدة الزعترية البشرية
|
|
|
|
|
|
|
مكتبة أمّ البنين النسويّة تصدر العدد 212 من مجلّة رياض الزهراء (عليها السلام)
|
|
|