
تاريخ الفيزياء

علماء الفيزياء


الفيزياء الكلاسيكية

الميكانيك

الديناميكا الحرارية


الكهربائية والمغناطيسية

الكهربائية

المغناطيسية

الكهرومغناطيسية


علم البصريات

تاريخ علم البصريات

الضوء

مواضيع عامة في علم البصريات

الصوت


الفيزياء الحديثة


النظرية النسبية

النظرية النسبية الخاصة

النظرية النسبية العامة

مواضيع عامة في النظرية النسبية

ميكانيكا الكم

الفيزياء الذرية

الفيزياء الجزيئية


الفيزياء النووية

مواضيع عامة في الفيزياء النووية

النشاط الاشعاعي


فيزياء الحالة الصلبة

الموصلات

أشباه الموصلات

العوازل

مواضيع عامة في الفيزياء الصلبة

فيزياء الجوامد


الليزر

أنواع الليزر

بعض تطبيقات الليزر

مواضيع عامة في الليزر


علم الفلك

تاريخ وعلماء علم الفلك

الثقوب السوداء


المجموعة الشمسية

الشمس

كوكب عطارد

كوكب الزهرة

كوكب الأرض

كوكب المريخ

كوكب المشتري

كوكب زحل

كوكب أورانوس

كوكب نبتون

كوكب بلوتو

القمر

كواكب ومواضيع اخرى

مواضيع عامة في علم الفلك

النجوم

البلازما

الألكترونيات

خواص المادة


الطاقة البديلة

الطاقة الشمسية

مواضيع عامة في الطاقة البديلة

المد والجزر

فيزياء الجسيمات


الفيزياء والعلوم الأخرى

الفيزياء الكيميائية

الفيزياء الرياضية

الفيزياء الحيوية

الفيزياء العامة


مواضيع عامة في الفيزياء

تجارب فيزيائية

مصطلحات وتعاريف فيزيائية

وحدات القياس الفيزيائية

طرائف الفيزياء

مواضيع اخرى
Isothermal Compression and Adiabatic Expansion of Ideal Gas
المؤلف:
Sidney B. Cahn, Gerald D. Mahan And Boris E. Nadgorny
المصدر:
A GUIDE TO PHYSICS PROBLEMS
الجزء والصفحة:
part 2 , p 15
30-8-2016
1991
Isothermal Compression and Adiabatic Expansion of Ideal Gas
An ideal gas is compressed at constant temperature τ from volume V1 to volume V2 (see Figure 1.1).
a) Find the work done on the gas and the heat absorbed by the gas.
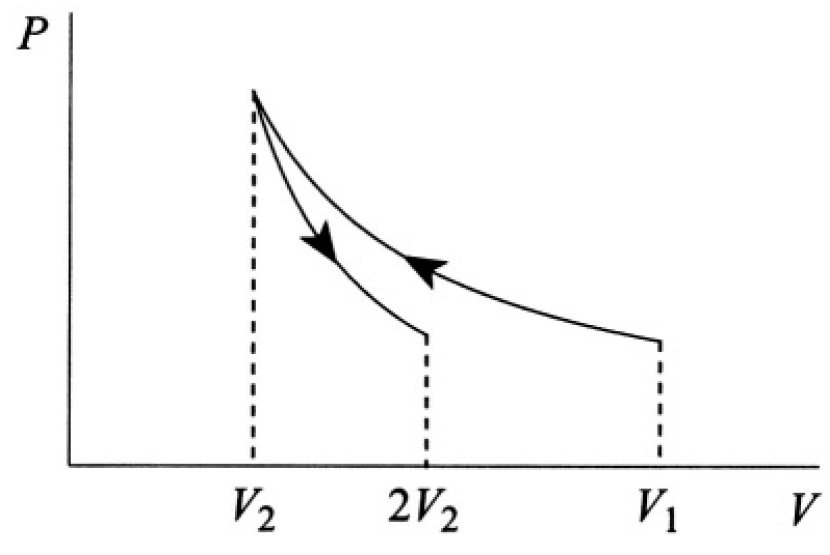
Figure 1.1
b) The gas now expands adiabatically to volume 2V2. What is the final temperature Tf (derive this result from first principles)?
c) Estimate Tf for Ti = 300 K for air.
SOLUTION
We can a) calculate the work as an integral, using the ideal gas law:
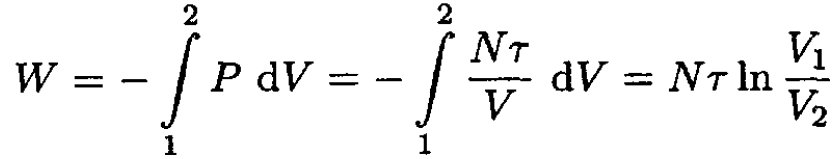 (1)
(1)
where τ is, as usual, the absolute temperature. Graphically, it is simply the area under the curve (see Figure 1.2). Alternatively, we can say that the work done is equal to the change of free energy F of the system:
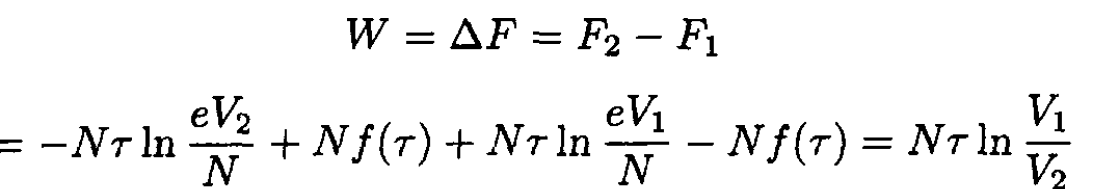 (2)
(2)

Figure 1.2
The total energy ε of the ideal gas depends only on the temperature, which is constant, so the heat absorbed by the gas is
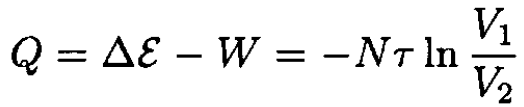 (3)
(3)
i.e., heat is rejected from the gas into the reservoir. Alternatively, since δQ = τ dS,
 (4)
(4)
the same result as in (3).
b) For an adiabatic expansion the entropy is conserved, so
 (5)
(5)
On the other hand,
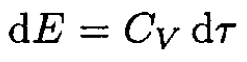 (6)
(6)
where CV is the specific heat for an ideal gas at constant volume. From (5) and (6), and using the ideal gas law, we obtain
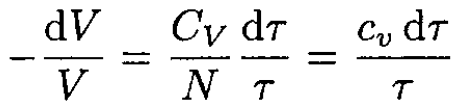 (7)
(7)
where CV/N ≡ cv, the specific heat per one molecule. Integrating (7) yields
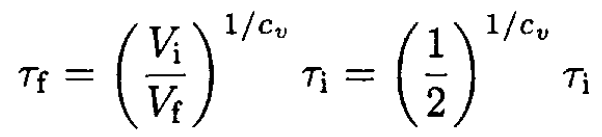 (8)
(8)
c) For air we may take cv = 5/2 (in regular units, cv = 5kB/2, it is mostly diatomic). Therefore,
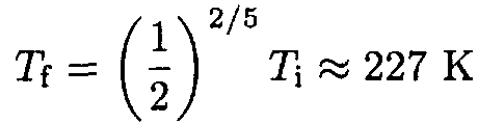
 الاكثر قراءة في مواضيع اخرى
الاكثر قراءة في مواضيع اخرى
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام) قسم الشؤون الفكرية يصدر مجموعة قصصية بعنوان (قلوب بلا مأوى)
قسم الشؤون الفكرية يصدر مجموعة قصصية بعنوان (قلوب بلا مأوى)


















