
Cylinder and Heat Bath
 المؤلف:
Sidney B. Cahn, Gerald D. Mahan And Boris E. Nadgorny
المؤلف:
Sidney B. Cahn, Gerald D. Mahan And Boris E. Nadgorny
 المصدر:
A GUIDE TO PHYSICS PROBLEMS
المصدر:
A GUIDE TO PHYSICS PROBLEMS
 الجزء والصفحة:
part 2 , p 16
الجزء والصفحة:
part 2 , p 16
 25-8-2016
25-8-2016
 1635
1635
Cylinder and Heat Bath
Consider a cylinder 1 m long with a thin, massless piston clamped in such a way that it divides the cylinder into two equal parts. The cylinder is in a large heat bath at T = 300K. The left side of the cylinder contains 1 mole of helium gas at 4 atm. The right contains helium gas at a pressure of 1 atm. Let the piston be released.
a) What is its final equilibrium position?
b) How much heat will be transmitted to the bath in the process of equilibration? (Note that R = 8.3 J/mole K).
SOLUTION
a) Since the process takes place at constant temperature, PV is constant for each side of the piston. When the piston is released, we can write
 (1)
(1)
where P01 and P0r are the initial pressures on the left and right sides of the cylinder, respectively, P is the final pressure on both sides of the cylinder, and V1 and Vr are the final volumes. From (1), we have
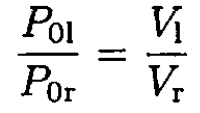 (2)
(2)
or
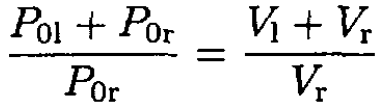 (3)
(3)
Therefore,
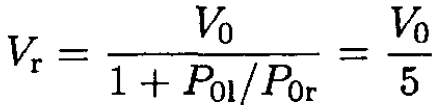 (4)
(4)
So, the piston winds up 20 cm from the right wall of the cylinder.
b) The energy of the ideal gas does not change in the isothermal process, so all the work done by the gas goes into heating the reservoir. Denoting by n1 and nr the number of moles on the left and right sides of the cylinder, respectively, and using nr = 1/4 mole, we obtain the total work and, hence, heat given by the integral
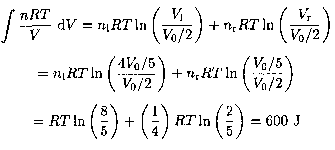
 الاكثر قراءة في مواضيع اخرى
الاكثر قراءة في مواضيع اخرى
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


