


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 8-3-2019
Date: 19-12-2015
Date: 3-1-2017
|
Microstates
In discussing the spectra of octahedral d2 complexes, we have emphasized the physical principles underlying the number of bands in the electronic spectrum. We now present a more formal treatment involving the construction of a table of microstates . Table 1.1 shows all possible combinations of two unpaired d electrons; for parallel spins, the terms must be triplets (S = 1) and in addition to the 3F ground state, the only excited state is the 3P. It can be shown from group theory that in an octahedral field, D, F and G, but not S and P, terms split.
Term Components in an octahedral field
S A1g
P T1g
D Eg +T2g
F A2g + T2g + T1g
G A1g + Eg + T2g + T1g
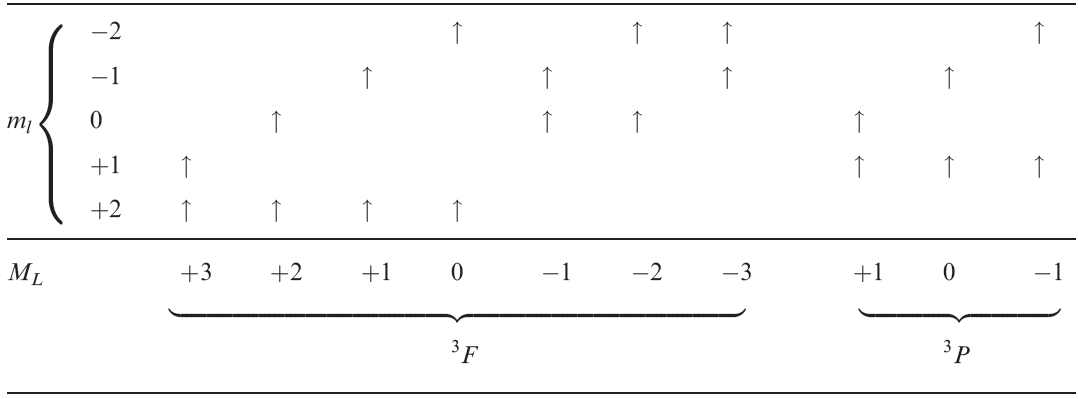
Similar splittings occur in a tetrahedral field, but the g labels given above are no longer applicable. For a d2 ion in an octahedral field, the possible triplet terms are therefore 3A2g, 3T2g and 3T1g derived from the triplet 3F ground term, and the 3T1 derived from the triplet 3P excited term.



|
|
|
|
تفوقت في الاختبار على الجميع.. فاكهة "خارقة" في عالم التغذية
|
|
|
|
|
|
|
أمين عام أوبك: النفط الخام والغاز الطبيعي "هبة من الله"
|
|
|
|
|
|
|
قسم شؤون المعارف ينظم دورة عن آليات عمل الفهارس الفنية للموسوعات والكتب لملاكاته
|
|
|