


 الفيزياء الكلاسيكية
الفيزياء الكلاسيكية
 الكهربائية والمغناطيسية
الكهربائية والمغناطيسية
 علم البصريات
علم البصريات
 الفيزياء الحديثة
الفيزياء الحديثة
 النظرية النسبية
النظرية النسبية
 الفيزياء النووية
الفيزياء النووية
 فيزياء الحالة الصلبة
فيزياء الحالة الصلبة
 الليزر
الليزر
 علم الفلك
علم الفلك
 المجموعة الشمسية
المجموعة الشمسية
 الطاقة البديلة
الطاقة البديلة
 الفيزياء والعلوم الأخرى
الفيزياء والعلوم الأخرى
 مواضيع عامة في الفيزياء
مواضيع عامة في الفيزياء|
Read More
Date: 22-8-2016
Date: 13-7-2016
Date: 16-3-2021
|
Ionizing Deuterium
The hydrogen atom has an ionization energy of EH = 13.5983 eV when an electron is bound to a proton. Calculate the ionization energy of deuterium: an electron bound to a deuteron. Give your answer as the difference between the binding energy of deuterium and hydrogen (δE = ED – EH). The deuteron has unit charge. The three masses are, in atomic mass units, me = 5.4858 × 10-4, mp = 1.00728, md = 2.01355.
SOLUTION
The ionization energy of hydrogen is just the binding energy of the electron which is given in terms of the reduced mass μep of the electron–proton system. The same expression for deuterium contains the reduced mass μed of the electron–deuteron system:
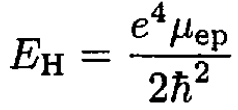 (1)
(1)
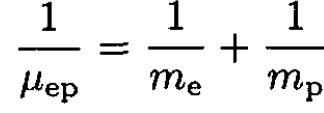 (2)
(2)
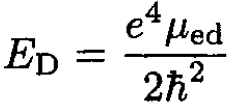 (3)
(3)
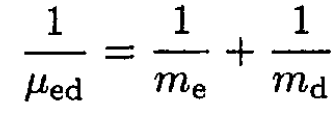 (4)
(4)
The difference is easily evaluated. The ratio me/mp is a small number and can be used as an expansion parameter:
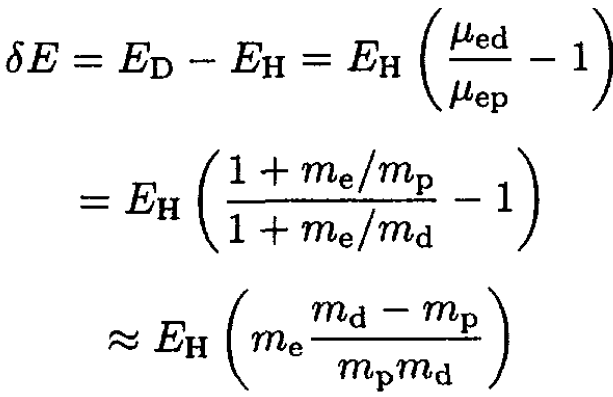 (5)
(5)
The ratio of masses gives 2.72 × 10-4 and δE ≈ 3.700 meV.



|
|
|
|
علامات بسيطة في جسدك قد تنذر بمرض "قاتل"
|
|
|
|
|
|
|
أول صور ثلاثية الأبعاد للغدة الزعترية البشرية
|
|
|
|
|
|
|
مكتبة أمّ البنين النسويّة تصدر العدد 212 من مجلّة رياض الزهراء (عليها السلام)
|
|
|