


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 14-10-2019
Date: 11-5-2017
Date: 18-11-2019
|
Resonance Delocalization in BH+ Type Acids
Now we will consider the effects of resonance delocalization of BH+ type acids and their neutral conjugate bases (B:). Comparing the pKa values of two ammonium acids (R–NH3+) we see that the example where R = Ph is significantly more acidic than when R = Me (Figure 1.1). This suggests that the aromatic ring provides significant stabilization of the conjugate base, which in this case is the neutral molecule aniline. Inspection of a resonance hybrid of aniline shows how the lone pair electrons on nitrogen delocalize into the aromatic ring (Figure1.2). With its electron density decreased, the N: atom in aniline is less basic than in methylamine and the corresponding anilinium ion (C6H5NH3+) is more acidic than the methylammonium ion (CH3NH3+).
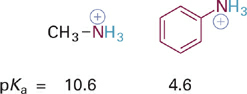
Figure 1.1 Example of increased acidity of +N–H acids with increasing resonance electron delocalization in the conjugate base.
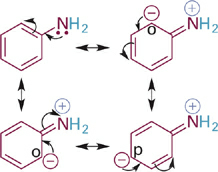
Figure 1.2 Resonance structures showing distribution of electron density among the N atom and the ortho- and para-carbon atoms of the phenyl ring.
In the examples above, we saw how delocalization of electrons from :X into a π system generally lowers the basicity of :X and makes the X–H bond more acidic. Now let’s examine some cases where resonance donation of electrons into a π system raises the basicity of :X and reduces acidity of the X–H bond of interest.
To illustrate this effect we will first compare the acidities of the guanidinium and iminium ions, which are BH+ type acids (Figure 5.15). In both cases, the N–H σ bond of interest involves an sp2 hybrid orbital on a nitrogen atom that is also involved in a C=N π bond. In the case of the iminium ion, the pKa simply reflects the intrinsic basicity of sp2-hybridized N. In the guanidinium ion, however, two N atoms are bound to carbon and each can contribute its lone pair electrons into the C=N π bond. The easiest way to see this is to examine a resonance hybrid structure of the guanidinium ion (Figure 1.3). As you can see, the effect of this resonance donation is to distribute the positive charge among all three nitrogen atoms. This greatly stabilizes the guanidinium ion, making it less prone to give up a proton and so much less acidic (pKa = ~13–14) than the iminium ion. From the perspective of the conjugate bases, we might say that guanidine is a much stronger base than an imine because it can more readily stabilize the positive charge that comes with attaining a proton.
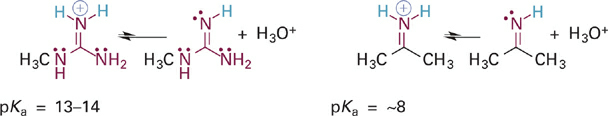
Figure 1.3 Acid-base reactions and pKa values for the guanidinium ion (left) and an iminium ion (right).
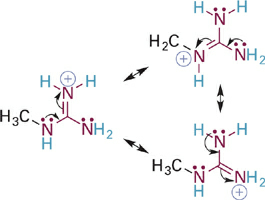
Figure 1.4 Resonance structures showing distribution of positive charge among all three N atoms of the guanidinium ion.
Another example of the same effect is apparent in a comparison of the relative acidities of the aromatic imidazolium and pyridinium ions (Figure 1.5). Here again the acidic N–H bond involves an sp2 hybrid orbital that lies in the plane of the ring. However, the second nitrogen atom in the imidazole ring does contribute its lone pair electrons into the π system, to satisfy Hückel’s rule (Figure 1.6). This resonance donation makes imidazole more basic than pyridine. By the same token, pyridinium is a stronger acid because it cannot stabilize a positive charge in the way the imidazolium ion does—by sharing charge between its two nitrogen atoms. It might help to draw a resonance hybrid of the imidazolium ion to convince yourself of this!
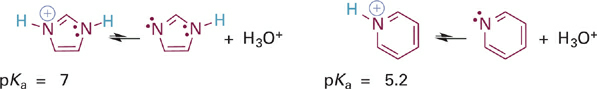
Figure 1.5 Acid-base reactions and pKa values for the imidazolium ion (left) and the pyridinium ion (right).
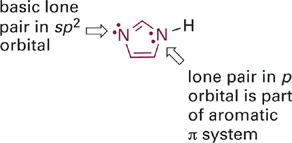
Figure 1.6 Lone pairs of electrons in different types of orbitals have different basicities. Lone pairs in sp2-orbitals are basic and can be protonated. Lone pairs in p-orbitals that are part of an aromatic π system are not basic and do not become protonated.



|
|
|
|
التوتر والسرطان.. علماء يحذرون من "صلة خطيرة"
|
|
|
|
|
|
|
مرآة السيارة: مدى دقة عكسها للصورة الصحيحة
|
|
|
|
|
|
|
نحو شراكة وطنية متكاملة.. الأمين العام للعتبة الحسينية يبحث مع وكيل وزارة الخارجية آفاق التعاون المؤسسي
|
|
|