


 الفيزياء الكلاسيكية
الفيزياء الكلاسيكية
 الكهربائية والمغناطيسية
الكهربائية والمغناطيسية
 علم البصريات
علم البصريات
 الفيزياء الحديثة
الفيزياء الحديثة
 النظرية النسبية
النظرية النسبية
 الفيزياء النووية
الفيزياء النووية
 فيزياء الحالة الصلبة
فيزياء الحالة الصلبة
 الليزر
الليزر
 علم الفلك
علم الفلك
 المجموعة الشمسية
المجموعة الشمسية
 الطاقة البديلة
الطاقة البديلة
 الفيزياء والعلوم الأخرى
الفيزياء والعلوم الأخرى
 مواضيع عامة في الفيزياء
مواضيع عامة في الفيزياء|
Read More
Date: 18-9-2020
Date: 18-5-2017
Date: 24-5-2016
|
Solids and their Behaviour
As has been said, solids are essentially rigid structures in which the particles vibrate about localized positions in an ordered array (often some form of crystal lattice). This perspective on the structure of a solid enables a number, but not all, of the different characteristics of solid matter to be understood.
Rigidity and Elasticity. Although solids are essentially rigid, most can be stretched, squashed and twisted. In such processes the component molecules are displaced from their equilibrium positions. Here, incidentally, it should be noted that, because of the shape of the intermolecular potential energy curve, it is much harder to squash a solid than to stretch it. In terms of the valley analogy it is harder to make lateral progress climbing the steep mountain than it is climbing the low hill. If the forces producing such distortions are removed then, provided the distortion is not too great, the solid will revert back to its original shape; the interparticle forces bring the particles ‘back into line’. The extent to which a solid is distorted by a given external force is, as might be expected, generally proportional to the strength of the force, the constant of proportionality being known as an elastic modulus. For solids, three such moduli are measured. They give information about the extent to which a solid can be stretched, twisted or squashed. Their values can also be used to estimate the strength of the relevant interparticle forces. Elastic properties of solids vary widely and an extreme situation is that of rubber which consists of molecules in the form of very long chains of carbon and hydrogen atoms. In equilibrium these chains are in a ‘higgledy-piggledy’ curled-up state and the striking elastic properties of rubber result from the way in which these long chain molecules can be elongated when the rubber is stretched and then revert to the original shape when released. If a solid is distorted too much then it will break. However there is much variation in how this comes about. Some metals (known as ductile), for example, can be distorted very much, and will retain that distortion, before finally breaking. Other metals (e.g. cast iron or glass) are brittle and after small distortion suddenly break. Then, again, plastic materials and rubber, which consist of long chain molecules that can slide over each other, can be stretched very considerably before breaking.
Thermal Expansion. When a solid is heated it expands and this can again be understood in terms of the shape of the potential energy curve. It has been mentioned that particles in a solid are vibrating about their equilibrium position. The higher the temperature the more energy this motion has and so the higher the system can climb up the potential energy curve. For a given energy the extremes of the motion are limited by the shape of the curve as shown in the figure. Because of the lopsided nature of the curve (steep mountain and low hill) it is clear that the higher the energy the greater is the average separation (centre point of the vibration). Hence, expansion takes place.
Melting. The phenomenon of melting (or fusion) is not well understood in detail but can be associated with the fact that as the temperature of a solid is increased so the amplitude of the particle vibrations increases. This means that a ‘loosening-up’ of the solid is taking place and it is at least qualitatively understandable that it makes a transition to the liquid state-what is called a phase change. When a solid reaches the melting temperature it remains at that temperature whilst the melting takes place and there is no
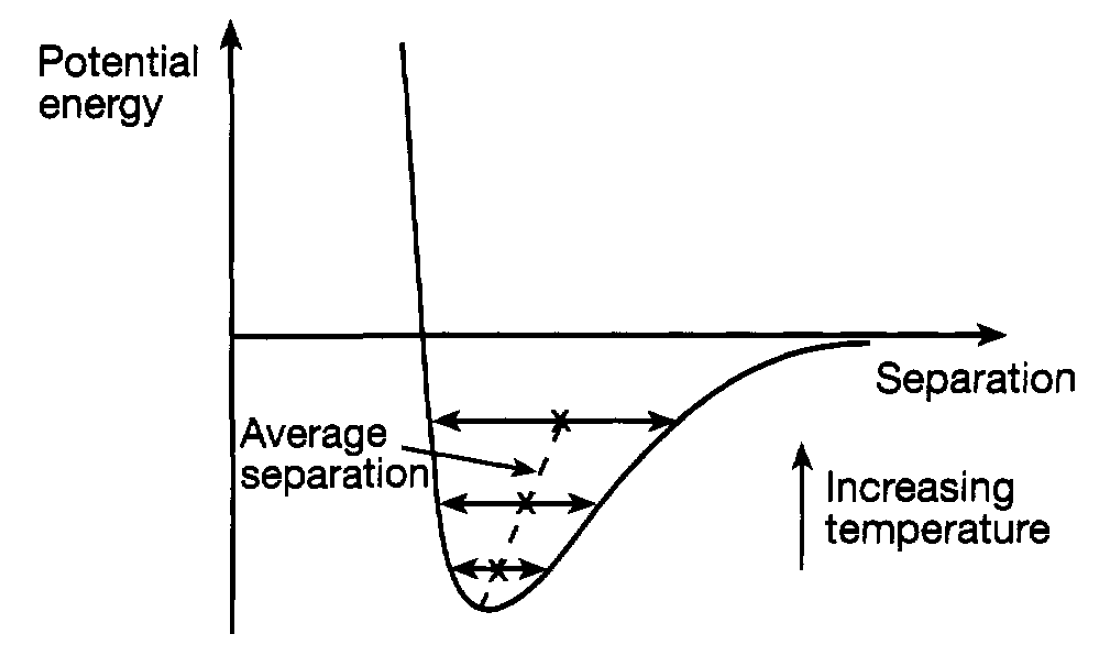
Figure 1.1: Vibration amplitude for different energies.
rise in temperature until the melting is completed. The heat provided to melt a solid is known as the latent heat of fusion. It has a much smaller value than the latent heat of sublimation, which is the heat needed to change a solid directly into a vapour. This is simply because, whilst in the liquid state the particles are in and out of the potential well, in the gaseous state they are completely separated and all have had to receive sufficient energy to surmount the depth of the potential well (the low hill).
Thermal Conductivity. This is the process in which heat energy is conducted through a substance and can be understood qualitatively as follows. Suppose a small region of a solid is heated. The temperature will rise and the molecules in that region will vibrate with increasing violence and amplitude. Because the neighbouring molecules feel the force of attraction of these agitated molecules they will be dragged into more violent motion themselves and so on through the solid. The disturbance will therefore be transmitted through the solid, molecules further and further away from the point of heat input will vibrate more energetically and the temperature there will therefore rise. In its travels the disturbance will meet obstacles, for example the surface of the solid, or imperfections and impurities, and taking the effect of these into account determines how good a conductor of heat a solid is. However, in dealing with metals, which are usually much better conductors of heat than non-metals, there are clearly other factors in play to account for this. These relate to the actual structure of the component atoms and to the fact that metals are also good conductors of electricity. This aspect of thermal conductivity is referred.



|
|
|
|
تفوقت في الاختبار على الجميع.. فاكهة "خارقة" في عالم التغذية
|
|
|
|
|
|
|
أمين عام أوبك: النفط الخام والغاز الطبيعي "هبة من الله"
|
|
|
|
|
|
|
قسم شؤون المعارف ينظم دورة عن آليات عمل الفهارس الفنية للموسوعات والكتب لملاكاته
|
|
|