


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 17-11-2020
Date: 14-12-2015
Date: 18-11-2020
|
Acetyl Coenzyme A
Acetyl coenzyme A (acetylCoA ) consists of a two-carbon activated acetyl unit attached to coenzyme A in thioester linkage. AcetylCoA is central to energy generation from the degradative pathways of oxidative fuel metabolism and to a number of biosynthetic pathways that utilize the activated two-carbon acetyl unit. In aerobic cells, it is the product of all the major catabolic pathways of fuel metabolism, including b- oxidation of fatty acids, ketone body degradation, glycolysis and pyruvate oxidation, ethanol oxidation, and the oxidative degradation of many amino acids. The two-carbon acetyl unit of acetylCoA formed from these pathways can be completely oxidized to CO2 in the tricarboxylic acid cycle (TCA cycle), thus providing aerobic cells with energy from the complete oxidation of fuels. The acetyl unit of acetylCoA is also the basic building block of fatty acids, cholesterol, and other compounds, and it can be transferred to other molecules in acetylation reactions (eg, synthesis of N-acetylated sugars).
1. Structure
The ability of acetylCoA to participate in these diverse metabolic pathways is derived from the thioester bond formed between the acyl carbon of the acetyl unit and the sulfhydryl group of coenzyme A (CoASH) (Fig. 1). Because sulfur does not share its electrons, the carbonyl carbon of the thioester bond carries a more positive partial charge than that of an oxygen ester, and electrons are pulled away from the C-H bonds of the terminal methyl group. This distribution of charge facilitates nucleophilic attack at the carbonyl carbon and enhances the ability of the terminal methyl carbon to act as an electrophilic agent in condensation reactions. The acetylCoA thioester bond is a high energy bond, with a DGO’ for hydrolysis of –32.2 kJ/mol. Transfer of the acetyl unit to other molecules, therefore, usually occurs with the release of energy.
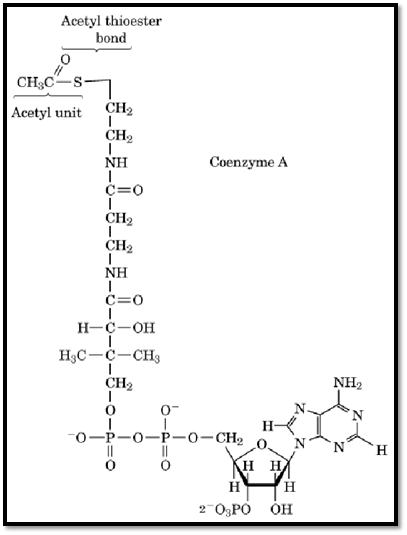
Figure 1. Structure of acetyl coenzyme A (acetyl CoA).
2. Formation of AcetylCoA in Oxidative Pathways
Three basic types of reactions exist in the oxidative pathways of fuel metabolism that generate acetylCoA: the activation of acetate, the thiolytic cleavage of b-ketoacyl CoAs and b-hydroxy acids, and the oxidative decarboxylation of pyruvate (Figure 2). In mammalian cells, acetate is the end product of ethanol metabolism and of threonine degradation. It is activated to acetylCoA in a single ATP- requiring step by the enzyme acetylCoA synthetase (Table 1). In an alternate route, bacterial cells can synthesize acetyl phosphate from acetate and then transfer the activated acetyl group to CoASH. Thiolytic cleavage of b-ketoacyl or b-hydroxy acylCoA derivatives to acetylCoA occurs in the pathways for oxidation of fatty acids, synthesis of the ketone bodies acetoacetate and b-hydroxybutyrate, and oxidative degradation of the amino acids isoleucine, leucine, lysine, tryptophan, phenylalanine, and tyrosine. (Most biochemistry textbooks contain general outlines of these pathways.) This type of reaction is illustrated by the b-oxidation spiral for fatty acids, in which one molecule of the C16-fatty acid palmitate is converted to eight molecules of acetylCoA by enzymes that sequentially oxidize the molecule to a b-ketoacyl compound and then cleave acetylCoA from the carboxylic acid end (Table 1). The third type of reaction, the oxidative decarboxylation of pyruvate by the pyruvate dehydrogenase complex, provides the connecting link between pathways that produce pyruvate and the TCA cycle. The inhibition of the pyruvate dehydrogenase complex by acetylCoA has a regulatory role in controlling the flow of carbon into the various pathways of intermediary metabolism.
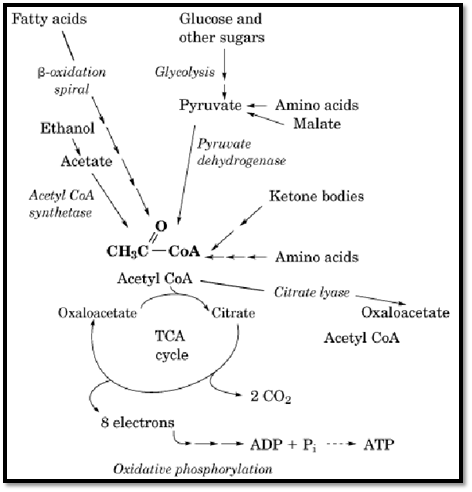
Figure 2. The role of acetyl CoA in oxidative fuel metabolism.
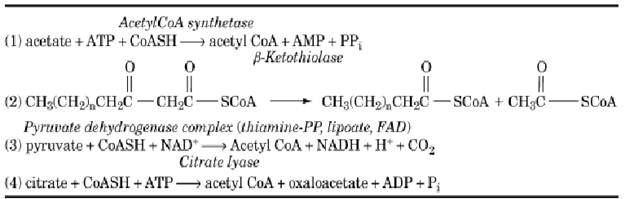
Table 1. Enzymes that Form Acetyl CoA
3. Oxidation of the Acetyl Unit of AcetylCoA in the TCA Cycle
It is estimated that about two thirds of the energy requirements of aerobic cells is met by the complete oxidation of the acetyl group of acetylCoA to CO2 in the TCA cycle (also called the citric acid cycle or the Krebs cycle.) In this cyclical sequence of reactions, acetylCoA condenses with oxaloacetate to form citrate in a reaction catalyzed by the enzyme citrate synthase. Subsequent reactions of the cycle donate electrons to the coenzymes NAD+ and FAD for ATP generation from oxidative phosphorylation, regenerate oxaloacetate, and release two carbons as CO2. The net reaction of the TCA cycle is:

Most of the pathways that produce acetylCoA in aerobic cells of eukaryotic organisms are located in the mitochondrial matrix, and most of the biosynthetic pathways that utilize acetylCoA are outside of the mitochondrion. AcetylCoA is not directly transported through the inner mitochondrial membrane but is “transferred” from the mitochondrial matrix to the cytosol as citrate. It is then regenerated in the cytosol by the enzyme citrate lyase.
4. AcetylCoA as a Precursor in Biosynthetic Reactions
The two-carbon acetyl unit of acetylCoA is the precursor of a number of compounds synthesized in cells. It is the basic building block of fatty acids, cholesterol, and other compounds derived from the five-carbon isoprenoid unit (Figure 3). In the synthesis of fatty acids, acetylCoA is carboxylated to malonylCoA by the biotin-requiring enzyme, acetylCoA carboxylase (Table 2). Subsequent reactions build the C16 fatty acid palmitate and other fatty acids from successive additions of the portion of malonyl CoA derived from acetylCoA, thereby providing the cell with the diverse fatty acids required for membrane lipids. In the synthesis of cholesterol, acetyl units from three acetylCoA molecules condense to form 3-hydroxy 3-methylglutaryl CoA (HMG CoA), which is subsequently decarboxylated and converted to the five-carbon isoprenoid unit isopentenyl pyrophosphate. This isoprenoid unit is one of the most common structural units of a number of compounds in mammalian cells, bacteria, and plants. AcetylCoA also contributes one carbon to compounds synthesized from the five-carbon intermediate a- ketoglutarate in the TCA cycle. The acetyl unit of acetylCoA can also
be transferred either to hydroxyl groups of compounds to form an acetyl oxygen ester (eg, the neurotransmitter acetylcholine; see Acetylcholine Receptor) or to an amino group to form an amide in an N-acetylation reaction (eg, acetylated amino sugars such as N-acetylglucosamine).
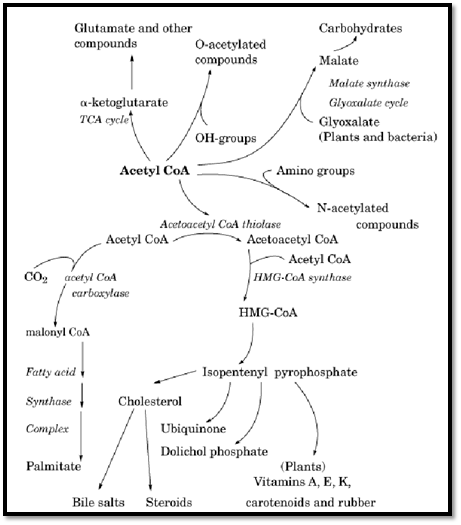
Figure 3. The central role of acetyl CoA in biosynthetic pathways.
Table 2. Enzymes That Utilize AcetylCoA
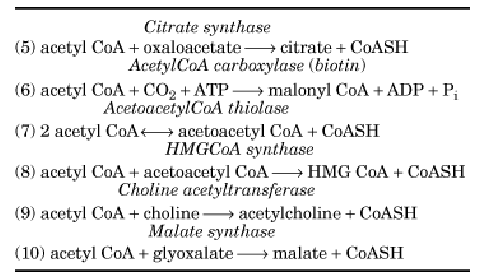
In mammalian cells, acetylCoA cannot provide a net source of carbon for the synthesis of glucose or other sugars. However, plants, yeast, and bacteria contain the glyoxylate cycle, which serves as a bypass of the TCA cycle. The net result of the glyoxylate cycle is the conversion of two molecules of acetylCoA to succinate, utilizing TCA cycle enzymes plus isocitrate lyase and malate synthase. As a result, Escherichia coli and many other bacteria are able to convert acetate to carbohydrates and amino acids and can thus utilize and grow on acetate as their sole carbon source.
References
A. L. Lehninger, D. L. Nelson, and M. M. Cox (1990) Principles of Biochemistry, 2nd ed., Worth Publishers, New York. Although the pathways mentioned above can be found in more detail in most textbooks, this book is particularly good for outlines of major pathways found in mammals, bacteria, and plants.
D. B. Marks, A. D. Marks, and C. M. Smith (1996) Basic Medical Biochemistry: A Clinical Approach, Williams & Wilkins, Baltimore, MD. Provides a general outline of pathways found in humans and their relationship to human physiologic and pathologic conditions.
R. H. Bethal, D. B. Buxtion, J. G. Robertson, and M. S. Olson (1993) Regulation of the pyruvate dehydrogenase multienzyme complex. Ann. Rev. Nutr. 13, 497–520. This article and the following one provide further information about the pyruvate dehydrogenase complex and acetylCoA carboxylase, two of the key enzymes in acetylCoA metabolism, which are highly regulated and the subject of current research.
G. M. Mabrouk, I. M. Helmy, K. G. Thampy, and S. J. Wakil (1990) Acute hormonal control of acetylCoA carboxylase: the roles of insulin, glucagon, and epinephrine. J. Biol. Chem. 265, 6330-6338
J. L. Goldstein, H. H. Hobbs, and M. S. Brown (1995) "Familial hypercholesterolemia". In The Metabolic and Molecular Bases of Inherited Disease, 7th ed. (C. R. Scriver, A. L. Beaudet, W. S. Sly, and D. Valle, eds.), McGraw Hill, New York: pp. 1981–2030. Covers certain aspects of pathway regulation for the synthesis of cholesterol from acetylCoA, which is of great interest for protection from heart disease.



|
|
|
|
دراسة يابانية لتقليل مخاطر أمراض المواليد منخفضي الوزن
|
|
|
|
|
|
|
اكتشاف أكبر مرجان في العالم قبالة سواحل جزر سليمان
|
|
|
|
|
|
|
المجمع العلمي ينظّم ندوة حوارية حول مفهوم العولمة الرقمية في بابل
|
|
|