


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
أقرأ أيضاً
التاريخ: 2024-02-28
التاريخ: 2024-05-04
التاريخ: 29-8-2019
التاريخ: 2023-07-31
|
The substitution method and the interconversion reactions discussed for proof of structure possibly may give you erroneous ideas about the reactions and reactivity of organic compounds. We certainly do not wish to imply that it is a simple, straightforward process to make all of the possible substitution products of a compound such as
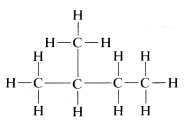
In fact, as will be shown later, direct substitution of bromine for hydrogen with compounds such as this does not occur readily, and when it does occur, the four possible substitution products indeed are formed, but in far from equal amounts because there are differences in reactivity for substitution at the different positions. Actually, some of the substitution products are formed only in very small quantities. Fortunately, this does not destroy the validity of the substitution method but does make it more difficult to apply. If direct substitution fails, some (or all) of the possible substitution products may have to be produced by indirect means. Nonetheless, you must understand that the success of the substitution method depends on determination of the total number of possible isomers - it does not depend on how the isomers are prepared.
Later, you will hear a lot about compounds or reagents being "reactive" and "unreactive." You may be exasperated by the loose way that these terms are used by organic chemists to characterize how fast various chemical changes occur. Many familiar inorganic reactions, such as the neutralization of hydrochloric acid with sodium hydroxide solution, are extremely fast at ordinary temperatures. But the same is not often true of reactions of organic compounds. For example, C2H5Br treated in two different ways is converted to gaseous compounds, one having the formula C2H6 and the other C2H4 . The C2H4 compound, ethene, reacts very quickly with bromine to give C2H4Br2 , but the C2H6 compound, ethane, does not react with bromine except at high temperatures or when exposed to sunlight (or similar intense light). The reaction products then are HBrHBr and C2H5Br, and later, HBr and C2H4Br2 , C2H3Br3 , and so on.
We clearly can characterize C2H4 as "reactive" and C2H6 as "unreactive" toward bromine. The early organic chemists also used the terms "unsaturated" and "saturated" for this behavior, and these terms are still in wide use today. But we need to distinguish between "unsaturated" and "reactive," and between "saturated" and "unreactive," because these pairs of terms are not synonymous. The equations for the reactions of ethene and ethane with bromine are different in that ethene adds bromine, C2H4+Br2→C2H4Br2, whereas ethane substitutes bromine, C2H6+Br2→C2H5Br+HBr.
You should reserve the term "unsaturated" for compounds that can, at least potentially, react by addition, and "saturated' for compounds that can only be expected to react by substitution. The difference between addition and substitution became much clearer with the development of the structure theory that called for carbon to be tetravalent and hydrogen univalent. Ethene then was assigned a structure with a carbon-to-carbon double bond, and ethane a structure with a carbon-to-carbon single bond:
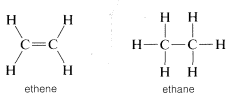
Addition of bromine to ethene subsequently was formulated as breaking one of the carbon-carbon bonds of the double bond and attaching bromine to these valences. Substitution was written similarly but here bromine and a C−H bond are involved:
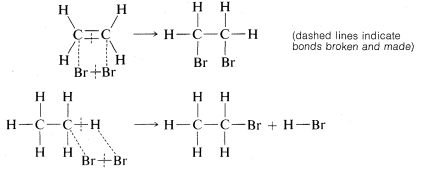
We will see later that the way in which these reactions actually occur is much more complicated than these simple equations indicate. In fact, such equations are regarded best as chemical accounting operations. The number of bonds is shown correctly for both the reactants and the products, and there is an indication of which bonds break and which bonds are formed in the overall process. However, do not make the mistake of assuming that no other bonds are broken or made in intermediate stages of the reaction.
Much of what comes later in this book will be concerned with what we know, or can find out, about the mechanisms of such reactions - a reaction mechanism being the actual sequence of events by which the reactants become converted to the products. Such information is of extraordinary value in defining and understanding the range of applicability of given reactions for practical preparations of desired compounds.
The distinction we have made between "unsaturated" and "reactive" is best illustrated by a definite example. Ethene is "unsaturated" (and "reactive") toward bromine, but tetrachloroethene, C2Cl4, will not add bromine at all under the same conditions and is clearly "unreactive." But is it also "saturated"?
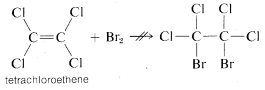
The answer is definitely no, because if we add a small amount of aluminum bromide, AlBr3, to a mixture of tetrachloroethene and bromine, addition does occur, although sluggishly:
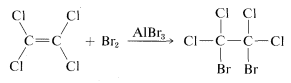
Obviously, tetrachloroethene is "unsaturated" in the sense it can undergo addition, even if it is unreactive to bromine in the absence of aluminum bromide.
The aluminum bromide functions in the addition of bromine to tetrachloroethene as a catalyst, which is something that facilitates the conversion of reactants to products. The study of the nature and uses of catalysts will concern us throughout this book. Catalysis is our principal means of controlling organic reactions to help form the product we want in the shortest possible time.
We will finesse here the long and important struggle of getting a truly self-consistent table of atomic weights. If you are interested in the complex history of this problem and the clear solution to it proposed by S. Cannizzaro in 1860, there are many accounts available in books on the history of chemistry. One example is J. R. Partington, A History of Chemistry, Vol. IV, Macmillan, London, 1964. Relative atomic weights now are based on 12C=12 (exactly).
Formulas such as this appear to have been used first by Crum Brown, in 1864, after the originators of structural formulas, A. Kekule and A. Couper (1858), came up with rather awkward, impractical representations. It seems incredible today that even the drawing of these formulas was severely criticized for many years. The pot was kept boiling mainly by H. Kolbe, a productive German chemist with a gift for colorful invective and the advantage of a podium provided by being editor of an influential chemical journal.



|
|
|
|
مقاومة الأنسولين.. أعراض خفية ومضاعفات خطيرة
|
|
|
|
|
|
|
أمل جديد في علاج ألزهايمر.. اكتشاف إنزيم جديد يساهم في التدهور المعرفي ؟
|
|
|
|
|
|
|
العتبة العباسية المقدسة تقيم ندوة علمية عن روايات كتاب نهج البلاغة
|
|
|