


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 16-3-2021
Date: 3-12-2015
Date: 28-4-2016
|
The Ribosome Influences the Accuracy of Translation
KEY CONCEPT
- The structure of the 16S rRNA at the P and A sites of the ribosome influences the accuracy of translation.
The error rate for incorporation of amino acids into polypeptides must be kept low, in the range of one misincorporation per 10,000 amino acids, to ensure that the functional properties of the encoded polypeptides are not altered in such a way as to be deleterious to the cell. Errors may be made in the following general stages of translation:
- Charging a tRNA only with its correct amino acid is clearly critical. This is a function of the aminoacyl-tRNA synthetase. The error rate varies with the particular enzyme, in the range of one misincorporation per 105 to 107 aminoacylations .
- Transporting only correctly aminoacylated tRNA to the ribosome, the function of initiation or elongation factors, can provide a mechanism for enhancing overall selectivity. In addition, these factors assist in the process of docking aminoacyl-tRNA to the ribosomal P and A sites.
- The specificity of codon–anticodon recognition is also crucial. Although binding constants vary with the individual codon–anticodon pairing, the intrinsic specificity associated with formation of a cognate versus noncognate 3-bp sequence (about 10-1 to 10-2 ) is far too low to provide an error rate of 10-5 .
It had long been assumed that the bacterial elongation factor EF-Tu is a sequence-nonspecific RNA-binding protein, given that it must transport all aminoacyl-tRNAs (except for the initiator tRNA) to the ribosome. However, EF-Tu recognizes both the amino acid portion of the aminoacyl-tRNA bond and the tRNA body, where it primarily binds to the sugar–phosphate backbone in the acceptor and T stems. Studies in which EF-Tu binding affinity to correctly and incorrectly aminoacylated tRNA was measured have shown that the strength of binding to the amino acid is inversely correlated with the strength of binding to the tRNA body; that is, weakly bound amino acids are correctly esterified to tightly bound tRNA bodies, and tightly bound amino acids are correctly esterified to weakly bound tRNA bodies. As a result, correctly acylated aminoacyl-tRNAs bind EF-Tu with quite similar affinities. Selectivity in overall translation can then result because misacylation of a weakly bound amino acid to a weakly bound tRNA body produces a noncognate aminoacyltRNA that interacts very poorly with EF-Tu. It is also possible that a misacylated aminoacyl-tRNA that binds more tightly to EF-Tu may
be discriminated against because it is more difficult to properly release this type upon docking to the ribosome.
It has been found that mutations in EF-Tu are able to suppress frameshifting errors . This implies that EF-Tu does not merely bring aminoacyl-tRNA to the A site, but it also is involved in positioning the incoming aminoacyl-tRNA relative to the peptidyl-tRNA in the P site. Similarly, mutations in the yeast initiation factor eIF2 allow the initiation of translation at a
start codon that is mutated from AUG to UUG. This implies a role for eIF2 in assisting the docking of tRNAiMet to the P site.
Proofreading on the ribosome, to enhance the intrinsically low level of specificity achievable from codon–anticodon base pairing alone, requires additional interactions provided by the local environment in the 30S subunit. In its function as a proofreader the ribosome amplifies the modest intrinsic selectivity of trinucleotide pairing by as much as 1,000-fold (FIGURE 1).
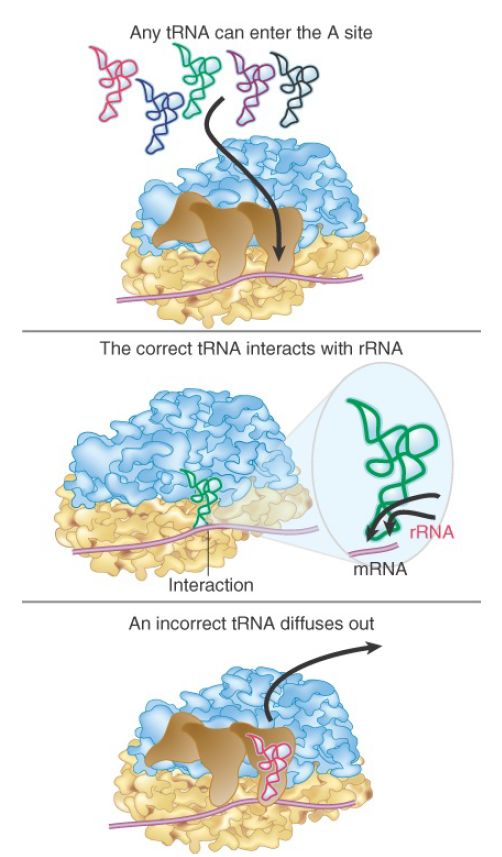
FIGURE 1. Any aminoacyl-tRNA can be placed in the A site (by EF-Tu), but only one that pairs with the anticodon can make stabilizing contacts with rRNA. In the absence of these contacts, the aminoacyl-tRNA diffuses out of the A site.
Aminoacyl-tRNA selection by the ribosome occurs at several stages along the pathway by which the EF-Tu–GTP–aminoacyltRNA ternary complex forms after aminoacylation delivers aminoacyl-tRNA to the ribosomal A site. First, a rather unstable initial binding complex forms with the ribosome. Next, there is a codon-recognition step in which the initial complex is rearranged to permit codon–anticodon pairing in the A site. Recall that the adjacent P site accommodates peptidyl-tRNA . Both the initial binding step and the subsequent codonrecognition step are reversible. Mispaired aminoacyl-tRNAs can be rejected at these stages by a combination of increased dissociation rates and/or lowered association rates for mispaired complexes.
After codon–anticodon recognition, a further conformational change triggers hydrolysis of GTP. Release of phosphate from the GDPbound EF-Tu then occurs; this release triggers another extensive conformational rearrangement, whereby EF-Tu–GDP dissociates from the aminoacyl-tRNA–ribosome complex. Only after EF-Tu dissociates do final conformational rearrangements associated with docking of the aminoacyl moiety into the 50S peptidyl transfer site, and the subsequent peptidyl transfer reaction, occur. In addition to selection at the early binding stage, rejection of mispaired aminoacyl-tRNA can also take place after the GTP hydrolysis step. Here the rejection occurs because the rate of the final conformational transition is very slow in the case of a misacylated complex. Thus, the overall specificity is enhanced because the tRNA must pass through two selection steps before peptide bond formation can occur.
The precision of codon–anticodon pairing in the A site is maintained by close monitoring of the steric and electrostatic properties of the trinucleotide. Three conserved bases in the 16S ribosomal RNA (A1492, A1493, and G530) interact closely with the minor groove of the codon–anticodon helix at the first two base pairs and are able to accurately assess the presence of canonical Watson–Crick base pairs at these positions. At the third (wobble) position, some noncanonical pairs can be accommodated because the ribosomal RNA does not monitor the pairing as closely. Ultimately, it is the failure of misacylated tRNA to fully meet the scrutiny of the ribosome at the codon–anticodon helix, and perhaps other positions, that leads to its rejection either before or after the GTP hydrolysis step.
Recently, an additional mechanism that contributes to the specificity of translation has been discovered: The ribosome is able to exert quality control after the formation of the peptide bond. In this mechanism, the formation of a peptide bond that arises from a mismatched aminoacyl-tRNA in the A site leads to a more general loss in specificity in the A site. In turn, this results in the early termination of translation.
The mechanism by which the ribosome recognizes errors after peptide bond synthesis is by monitoring the precise complementarity of the codon–anticodon helix in the peptidyl (P) site. The consequence of the misincorporation is the increased capacity of release factors to bind in the A site to cause premature termination, even when a stop codon is not present. Additionally, the rate of improper coding in the adjacent A site is increased. The resulting propagation of errors ultimately leads to premature termination.
The cost of translation, as calculated by the number of high-energy bonds that must be hydrolyzed, is clearly increased by proofreading processes. The extent of the increased energetic cost depends on the stage at which the misacylated tRNA is rejected.
The cost associated with rejection before GTP hydrolysis is associated only with the production of the misacylated tRNA by the tRNA synthetase. However, if GTP is hydrolyzed before the mismatched aminoacyl-tRNA dissociates, the energetic cost will be greater. Of course, the greatest cost is associated with the premature termination of translation to give a nonfunctional product, in post-peptidyl-transfer quality control. In that case, the full energetic payment associated with synthesis of the polypeptide to the point of premature release must be paid.



|
|
|
|
دخلت غرفة فنسيت ماذا تريد من داخلها.. خبير يفسر الحالة
|
|
|
|
|
|
|
ثورة طبية.. ابتكار أصغر جهاز لتنظيم ضربات القلب في العالم
|
|
|
|
|
|
|
سماحة السيد الصافي يؤكد ضرورة تعريف المجتمعات بأهمية مبادئ أهل البيت (عليهم السلام) في إيجاد حلول للمشاكل الاجتماعية
|
|
|