


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 30-3-2021
Date: 29-11-2015
Date: 17-11-2020
|
Small Subunits Scan for Initiation Sites on Eukaryotic mRNA
KEY CONCEPTS
- Eukaryotic 40S ribosomal subunits bind to the 5′ end of mRNA and scan the mRNA until they reach an initiation site.
- A eukaryotic initiation site consists of a 10-nucleotide sequence that includes an AUG codon.
- 60S ribosomal subunits join the complex at the initiation site.
Initiation of translation in eukaryotic cytoplasm resembles the process that occurs in bacteria, but the order of events is different and the number of accessory factors is greater. Some of the differences in initiation are related to a difference in the way that bacterial 30S and eukaryotic 40S subunits find their binding sites for initiating translation on mRNA. In eukaryotes, small subunits first recognize the 5′ cap at the end of the mRNA and then move to the initiation site, where they are joined by large subunits. (In prokaryotes, small subunits bind directly to the initiation site.)
Virtually all eukaryotic mRNAs are monocistronic, but each mRNA usually is substantially longer than the sequence that encodes its polypeptide. The average mRNA in eukaryotic cytoplasm is 1,000 to 2,000 bases long, has a methylated cap at the 5′ terminus, and carries 100 to 200 adenine bases at the 3′ terminus. The untranslated 5′ leader is relatively short, usually less than 100 bases. The length of the coding region is determined by the size of the polypeptide product. The untranslated 3′ trailer is often rather long, at times reaching lengths of up to about 1,000 bases.
The first feature to be recognized during translation of a eukaryoticmR NA is the methylated cap at the 5′ end. mRNAs whose caps have been removed are not translated efficiently in vitro. Binding of 40S subunits to mRNAs requires several initiation factors, including proteins that recognize the structure of the cap.
Modification at the 5′ end occurs in almost all cellular or viral mRNAs and is essential for their translation in eukaryotic cytoplasm (although it is not needed in mitochondria or chloroplasts). The sole exception to this rule is provided by a few viral mRNAs (such as those of poliovirus) that are not capped; only these exceptional viral mRNAs can be translated in vitro without caps. They use an alternative pathway that bypasses the need for the cap.
We have dealt with the process of initiation as though the initiation site is always freely available. However, its availability may be impeded by the mRNA’s secondary structure. The recognition of mRNA requires several additional factors; an important part of their function is to remove any secondary structure in the mRNA.
In some mRNAs, the AUG initiation codon lies within 40 bases of the 5′ terminus of the mRNA, so that both the cap and AUG lie within the span of ribosome binding. However, in many mRNAs the cap and AUG are farther apart; in extreme cases, they can be as much as 1,000 bases away from each other. Yet the presence of the cap is still necessary for a stable complex to be formed at the initiation codon. How can the ribosome rely on two sites so far apart for mRNA recognition?
Figure 1 illustrates the “scanning” model, which has the 40S subunit initially recognizing the 5′ cap and then “migrating” along the mRNA. Scanning from the 5′ end is a linear process. When 40S subunits scan the leader region, they can melt secondary structure hairpins with stabilities less than −30 kcal, but hairpins of greater stability impede or prevent migration.
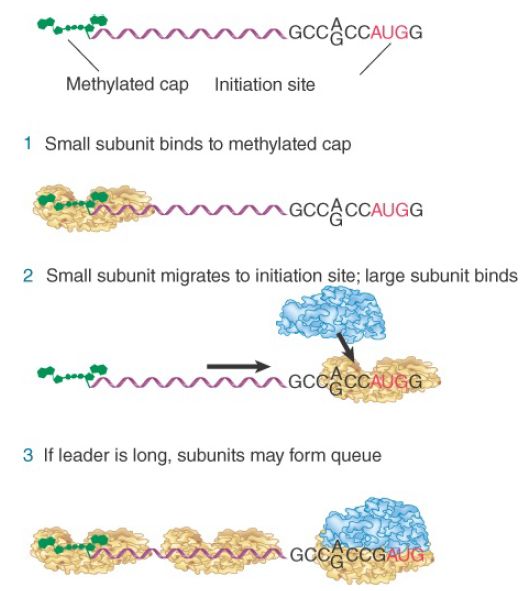
FIGURE 1. Eukaryotic ribosomes migrate from the 5′ end of mRNA to the ribosome binding site, which includes an AUG initiation codon.
Migration stops when the 40S subunit encounters the AUG initiation codon. Usually, though not always, the first AUG triplet sequence to be encountered will be the initiation codon. However, the AUG triplet by itself is not sufficient to halt migration; it is recognized efficiently as an initiation codon only when it is in the right context.
The most important determinants of context are the bases in positions −4 and +1. An initiation codon may be recognized in the sequence NNNPuNNAUGG by the small ribosomal subunit using the Met-tRNA anticodon. The purine (A or G) three bases before the AUG codon and the G immediately following it can influence the efficiency of translation by 10 times. When the leader sequence is long, further 40S subunits can recognize the 5′ end before the first has left the initiation site, creating a queue of subunits proceeding along the leader to the initiation site.
It is usually true that the initiation codon is the first AUG to be encountered in the most efficiently translated mRNAs. However, what happens when there is an AUG triplet in the 5′ untranslated region (UTR)? Two escape mechanisms are possible for a ribosome that starts scanning at the 5′ end. The most common isthat scanning is leaky; that is, a ribosome may continue past a noninitiation AUG because it is not in the right context. In the rare case that it does recognize the AUG, it may initiate translation but terminate before the proper initiation codon, after which it resumes scanning.
The majority of eukaryotic initiation events involve scanning from the 5′ cap, but there is an alternative means of initiation, used especially by certain viral RNAs, in which a 40S subunit associates directly with an internal site called an internal ribosome entry site (IRES). In this case, any AUG codons that may be in the 5′ UTR are bypassed entirely. There are few sequence homologies between known IRES elements. Three types of IRESs can be identified based on their interaction with the 40S subunit:
- The most common type of IRES includes the AUG initiation codon at its upstream boundary. The 40S subunit binds directly to it, using a subset of the same factors that are required for initiation at 5′ ends.
- Another type of IRES is located as much as 100 nucleotides upstream of the AUG, requiring a 40S subunit to migrate, again probably by a scanning mechanism. An exceptional type of IRES in hepatitis C virus can bind a 40S subunit directly, without requiring any initiation factors. The
order of events is different from all other eukaryotic initiation. Following 40S-mRNA binding, a complex containing initiator factors and the initiator tRNA binds.
Use of the IRES is especially important in picornavirus infection, where it was first discovered, because the virus inhibits host translation by destroying cap structures and inhibiting the initiation factors that bind them. One such target is subunit eIF4G , which binds the 5′ end of mRNA. Thus, infection prevents translation of host mRNAs but allows viral mRNAs to be translated because they use the IRES.
Ribosome binding is stabilized at the initiation site. When the 40S subunit is joined by a 60S subunit, the intact ribosome is located at the site identified by the protection assay. A 40S subunit protects a region of up to 60 bases; when the 60S subunits join the complex the protected region contracts to about the same length of 30 to 40 bases seen in prokaryotes.



|
|
|
|
التوتر والسرطان.. علماء يحذرون من "صلة خطيرة"
|
|
|
|
|
|
|
مرآة السيارة: مدى دقة عكسها للصورة الصحيحة
|
|
|
|
|
|
|
نحو شراكة وطنية متكاملة.. الأمين العام للعتبة الحسينية يبحث مع وكيل وزارة الخارجية آفاق التعاون المؤسسي
|
|
|