


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية| The 3′ Ends of mRNAs Are Generated by Cleavage and Polyadenylation |
|
|
|
Read More
Date: 29-11-2015
Date: 15-4-2021
Date: 30-11-2015
|
The 3′ Ends of mRNAs Are Generated by Cleavage and Polyadenylation
KEY CONCEPTS
- The sequence AAUAAA is a signal for cleavage to generate a 3′ end of mRNA that is polyadenylated.
- The reaction requires a protein complex that contains a specificity factor, an endonuclease, and poly(A) polymerase.
- The specificity factor and endonuclease cleave RNA downstream of AAUAAA.
- The specificity factor and poly(A) polymerase add about 200 A residues processively to the 3′ end.
- The poly(A) tail controls mRNA stability and influences translation.
- Cytoplasmic polyadenylation plays a role in Xenopus embryonic development.
It is not clear whether RNA polymerase II actually engages in a termination event at a specific site. It is possible that its termination is only loosely specified. In some transcription units, termination occurs more than 1,000 bp downstream of the site, corresponding to the mature 3′ end of the mRNA (which is generated by cleavage at a specific sequence). Instead of using specific terminator sequences, the enzyme ceases RNA synthesis within multiple sites located in rather long “terminator regions.” The nature of the individual termination sites is largely unknown.
The mature 3′ ends of Pol II transcribed mRNAs are generated by cleavage followed by polyadenylation. Addition of poly(A) to nuclear RNA can be prevented by the analog 3′–deoxyadenosine, which is also known as cordycepin. Although cordycepin does not stop the transcription of nuclear RNA, its addition prevents the appearance of mRNA in the cytoplasm. This shows that polyadenylation is necessary for the maturation of mRNA from nuclear RNA. The poly(A) tail is known to protect the mRNA from degradation by 3′–5′ exonucleases. In yeast, it is suggested that the poly(A) tail also plays a role in facilitating nuclear export of matured mRNA and in cap stability.
Generation of the 3′ end is illustrated in FIGURE 1. The RNA polymerase transcribes past the site corresponding to the 3′ end, and sequences in the RNA are recognized as targets for an
endonucleolytic cut followed by polyadenylation. RNA polymerase continues transcription after the cleavage, but the 5′ end that is generated by the cleavage is unprotected, which signals
transcriptional termination (see the next section, 3′ mRNA End Processing Is Critical for Termination of Transcription).

FIGURE 1. The sequence AAUAAA is necessary for cleavage to generate a 3′ end for polyadenylation.
The site of cleavage/polyadenylation in most pre-mRNAs is flanked by two cis-acting signals: an upstream AAUAAA motif, which is usually located 11 to 30 nucleotides from the site, and a
downstream U-rich or GU-rich element. The AAUAAA is needed for cleavage and polyadenylation because deletion or mutation of the AAUAAA hexamer prevents generation of the polyadenylated 3′ end (though in plants and fungi there can be considerable variation from the AAUAAA motif).
The development of a system in which polyadenylation occurs in vitro opened the route to analyzing the reactions. The formation and functions of the complex that undertakes 3′ processing are illustrated in FIGURE 2. Generation of the proper 3′ terminal structure depends on the cleavage and polyadenylation specific factor (CPSF), which contains multiple subunits. One of the subunits binds directly to the AAUAAA motif and to the cleavage stimulatory factor (CstF), which is also a multicomponent complex.
One of these components binds directly to a downstream GU-rich sequence. CPSF and CstF can enhance each other in recognizing the polyadenylation signals. The specific enzymes involved are an endonuclease (the 73-kD subunit of CPSF) to cleave the RNA and a poly(A) polymerase (PAP) to synthesize the poly(A) tail.
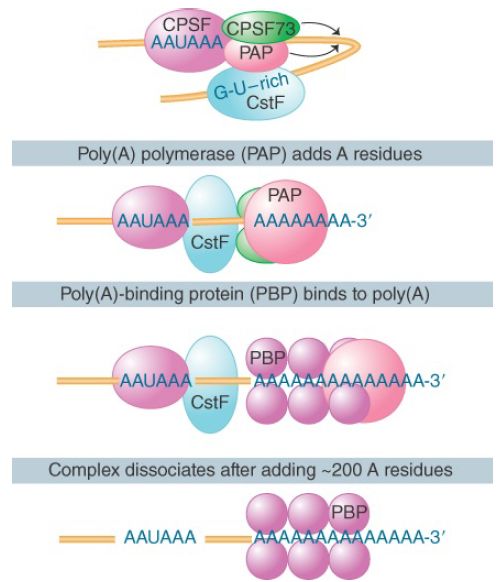
FIGURE 2. The 3′ processing complex consists of several activities. CPSF and CstF each consist of several subunits; the other components are monomeric. The total mass is more than 900 kD.
PAP has nonspecific catalytic activity. When it is combined with the other components, the synthetic reaction becomes specific for RNA containing the sequence AAUAAA. The polyadenylation reaction passes through two stages. First, a rather short oligo(A) sequence (about 10 residues) is added to the 3′ end. This reaction is absolutely dependent on the AAUAAA sequence, and poly(A) polymerase performs it under the direction of the specificity factor.
In the second phase, the nuclear poly(A) binding protein (PABP II) binds the oligo(A) tail to allow extension of the poly(A) tail to the full length of about 200 residues. The poly(A) polymerase by itself adds A residues individually to the 3′ position. Its intrinsic mode of action is distributive; it dissociates after each nucleotide has been added. However, in the presence of CPSF and PABP II it functions processively to extend an individual poly(A) chain. After the polyadenylation reaction, PABP II binds stoichiometrically to the poly(A) stretch, which by some unknown mechanism limits the action of poly(A) polymerase to about 200 additions of A residues.
Upon export of mature mRNAs out of the nucleus, the poly(A) tail is bound by the cytoplasmic poly(A) binding protein (PABP I). PABP I not only protects the mRNA from degradation by the 3′ to 5′ exonucleases but also binds to the translation initiation factor eIF4G to facilitate translation of the mRNA. Thus, the mRNA in the cytoplasm forms a closed loop in which a protein complex contains both the 5′ and 3′ ends of the mRNA . Polyadenylation therefore affects both stability and initiation of translation in the cytoplasm.
During embryonic development of Xenopus, polyadenylation is carried out in the cytoplasm to provide a maternal control in early embryogenesis. Some stored maternal mRNAs may either be polyadenylated by the poly(A) polymerase in the cytoplasm to stimulate translation or deadenylated to terminate translation. A specific AU-rich cis-acting element (CPE) in the 3′ tail directs the meiotic maturation-specific polyadenylation in the cytoplasm to activate translation of some specific maternal mRNAs. To regulate mRNA degradation, at least two types of cis-acting sequences in the 3′ tail can trigger mRNA deadenylation: embryonic deadenylation element (EDEN), a 17-nucleotide sequence, and ARE elements, which are AU rich, usually containing tandem repeats of AUUUA. A poly(A)-specific RNAase (PARN) is involved in mRNA degradation in the cytoplasm. Of course, mRNA deadenylation is always in competition with mRNA stabilization, which together determine the half-life of individual mRNAs in the cell.



|
|
|
|
التوتر والسرطان.. علماء يحذرون من "صلة خطيرة"
|
|
|
|
|
|
|
مرآة السيارة: مدى دقة عكسها للصورة الصحيحة
|
|
|
|
|
|
|
نحو شراكة وطنية متكاملة.. الأمين العام للعتبة الحسينية يبحث مع وكيل وزارة الخارجية آفاق التعاون المؤسسي
|
|
|