


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 15-3-2021
Date: 21-11-2020
Date: 7-5-2021
|
P Elements Are Activated in the Germline
KEY CONCEPTS
- P elements are activated in the germline of P male × M female crosses because a tissue-specific splicing event removes one intron, which generates the coding sequence for the transposase.
- The P element also produces a repressor of transposition, which is inherited maternally in the
cytoplasm.
- The presence of the repressor explains why M male × P female crosses remain fertile.
Activation of P elements is tissue specific: It occurs only in the germline. P elements are transcribed, though, in both germline and somatic tissues. Tissue specificity is conferred by a change in the splicing pattern.
FIGURE 1. depicts the organization of the element and its transcripts. The primary transcript extends for 2.5 or 3.0 kb, the difference probably reflecting merely the leakiness of the termination site. Two protein products can be produced:
- In somatic tissues, only the first two introns are excised, creating a coding region of ORF0-ORF1-ORF2. Translation of this RNA yields a protein of 66 kD. This protein is a repressor of transposon activity.
- In germline tissues, an additional splicing event occurs to remove intron 3. This connects all four open reading frames into an mRNA that is translated to generate a protein of 87 kD. This protein is the transposase.
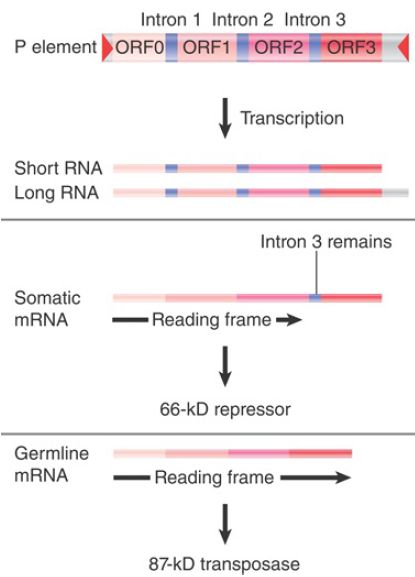
FIGURE 1.The P element has four exons. The first three are spliced together in somatic expression; all four are spliced together in germline expression.
Two types of experiments have demonstrated that splicing of the third intron is needed for transposition. First, if the splicing junctions are mutated in vitro and the P element is reintroduced into flies, its transposition activity is abolished. Second, if the third intron is deleted, so that ORF3 is constitutively included in the mRNA in all tissues, transposition occurs in somatic tissues as well as the germline. Thus, whenever ORF3 is spliced to the preceding reading frame, the P element becomes active. This is the crucial regulatory event, and usually it occurs only in the germline.
What is responsible for the tissue-specific splicing? Somatic cells contain a protein that binds to sequences in exon 3 to prevent splicing of the last intron . The absence of this protein in germline cells allows splicing to generate the mRNA that encodes the transposase.
Transposition of a P element requires about 150 bp of terminal DNA. The transposase binds to 10-bp sequences that are adjacent to the 31-bp inverted repeats. Transposition occurs by a nonreplicative cut-and-paste mechanism resembling that of Tn10. It contributes to hybrid dysgenesis in two ways: Insertion of the transposed element at a new site may cause mutations, and the break that is left at the donor site can have a deleterious effect.
It is interesting that, in a significant proportion of cases, the break in donor DNA is repaired by using the sequence of the homologous chromosome. If the homolog has a P element, the presence of a P element at the donor site may be restored (so the event resembles the result of a replicative transposition). If the homolog lacks a P element, repair may generate a sequence lacking the P element, thus apparently providing a precise excision (an unusual event in other transposable systems).
The dependence of hybrid dysgenesis on the origin of the female in a cross shows that the cytoplasm is important, as are the P factors themselves. The contribution of the cytoplasm is described as the cytotype; a line of flies containing P elements has P cytotype, whereas a line of flies lacking P elements has M cytotype. Hybrid dysgenesis occurs only when chromosomes containing P factors find themselves in M cytotype; that is, when the male parent has P elements and the female parent does not.
Cytotype shows an inheritable cytoplasmic effect; when a cross occurs through P cytotype (the female parent has P elements), hybrid dysgenesis is suppressed for several generations of crosses with M female parents. Thus, something in P cytotype, which can be diluted out over some generations, suppresses hybrid dysgenesis.
The effect of cytotype has been a particularly puzzling phenomenon. All explanations assume that a repressor molecule is deposited into the egg cell cytoplasm, as illustrated in FIGURE 2. The repressor is provided as a maternal factor in the egg. In a P line, sufficient repressor must be present to prevent transposition from occurring, even though the P elements are present. In any cross involving a P female, its presence prevents either synthesis or activity of the transposase. When the female parent is M type, though, no repressor is present in the egg, and the introduction of a P element from the male parent results in activity of transposase in the germline. The ability of P cytotype to exert an effect through more than one generation suggests that there must be enough repressor protein in the egg, and that it must be stable enough, to be passed on through the adult to be present in the eggs of the next generation.
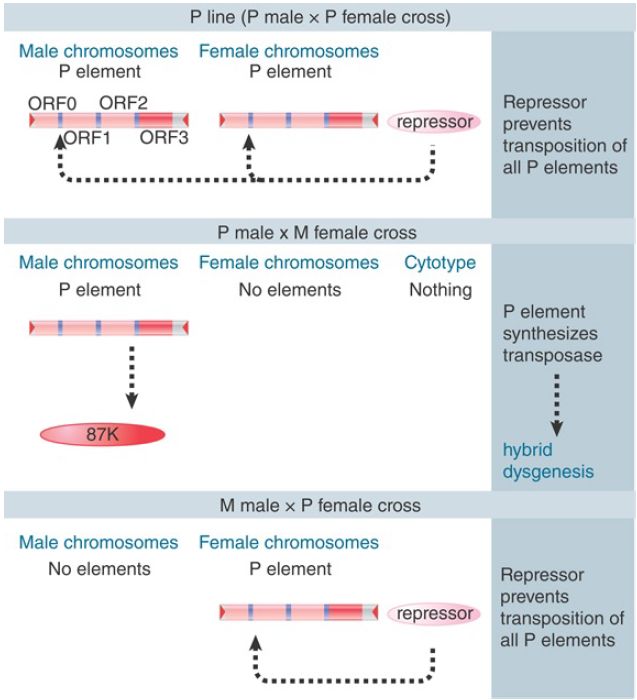
FIGURE 2. Hybrid dysgenesis is determined by the interactions between P elements in the genome and repressors in the cytotype.
For many years, the best candidate for the repressor was the 66-kD protein. However, some strains of flies lack P elements capable of producing a 66-kD repressor protein and yet still exhibit the P cytotype. More recent evidence has implicated small RNAs in P element repression; genes important in processing small RNAs derived from P element transcripts (and those of several other transposons as well) are also required for efficient transposon silencing. This observation has led to a model in which P cytotype is conditioned by P elements at particular positions that produce transcripts that are processed into a specific class of small RNAs called piRNAs . In this case, it is the presence of these small RNAs in the cytoplasm that are responsible for P element cytotype repression. Like the small RNAs involved in RNA interference, piRNAs are hypothesized to direct the degradation of P element transcript. An appealing feature of this model is that it suggests that P element cytotype repression is a particular example of a widespread mechanism by which transposon activity is repressed in plants, fungi, and animals.
Remarkably, P elements have only been detectable in the D. melanogaster genome for a few decades. They came from a second species of Drosophila, D. willisoni, through a horizontal transfer of P element sequence. Subsequent to that transfer, P elements rapidly spread throughout the worldwide population of D.melanogaster. Analysis of P elements in a variety of Drosophila species reveals that horizontal transfer of this transposon has occurred repeatedly throughout its history. This propensity to move between species has been documented among a number of transposons, leading to the suggestion that an important component to the transposon life cycle is the ability to regularly invade “naïve” genomes that lack sequences (such as those that produce piRNAs) that can repress transposon activity.



|
|
|
|
هل يمكن أن تكون الطماطم مفتاح الوقاية من السرطان؟
|
|
|
|
|
|
|
اكتشاف عرائس"غريبة" عمرها 2400 عام على قمة هرم بالسلفادور
|
|
|
|
|
|
|
جامعة الكفيل تقيم ندوة علمية عن الاعتماد الأكاديمي في جامعة جابر بن حيّان
|
|
|