
 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 24-1-2018
Date: 27-4-2019
Date: 5-5-2019
|
Uses of Lanthanides in Devices
Lanthanides (elements 57–71) are fairly abundant in the earth’s crust, despite their historic characterization as rare earth elements. Thulium, the rarest naturally occurring lanthanoid, is more common in the earth’s crust than silver (4.5 × 10−5% versus 0.79 × 10−5% by mass). There are 17 rare earth elements, consisting of the 15 lanthanoids plus scandium and yttrium. They are called rare because they were once difficult to extract economically, so it was rare to have a pure sample; due to similar chemical properties, it is difficult to separate any one lanthanide from the others. However, newer separation methods, such as ion exchange resins similar to those found in home water softeners, make the separation of these elements easier and more economical. Most ores that contain these elements have low concentrations of all the rare earth elements mixed together.
The commercial applications of lanthanides are growing rapidly. For example, europium is important in flat screen displays found in computer monitors, cell phones, and televisions. Neodymium is useful in laptop hard drives and in the processes that convert crude oil into gasoline (Figure 1.1 ). Holmium is found in dental and medical equipment. In addition, many alternative energy technologies rely heavily on lanthanoids. Neodymium and dysprosium are key components of hybrid vehicle engines and the magnets used in wind turbines.
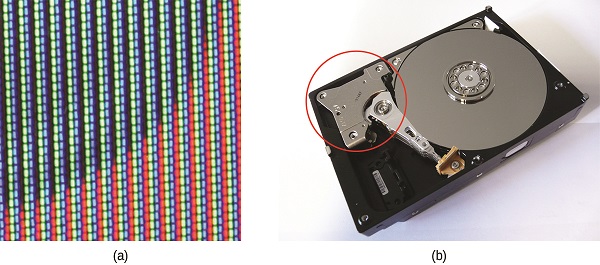
Figure 1.1 : (a) Europium is used in display screens for televisions, computer monitors, and cell phones. (b) Neodymium magnets are commonly found in computer hard drives. (credit b: modification of work by “KUERT Datenrettung”/Flickr)
As the demand for lanthanide materials has increased faster than supply, prices have also increased. In 2008, dysprosium cost $110/kg; by 2014, the price had increased to $470/kg. Increasing the supply of lanthanoid elements is one of the most significant challenges facing the industries that rely on the optical and magnetic properties of these materials.
The transition elements have many properties in common with other metals. They are almost all hard, high-melting solids that conduct heat and electricity well. They readily form alloys and lose electrons to form stable cations. In addition, transition metals form a wide variety of stable coordination compounds, in which the central metal atom or ion acts as a Lewis acid and accepts one or more pairs of electrons. Many different molecules and ions can donate lone pairs to the metal center, serving as Lewis bases. In this chapter, we shall focus primarily on the chemical behavior of the elements of the first transition series.



|
|
|
|
دخلت غرفة فنسيت ماذا تريد من داخلها.. خبير يفسر الحالة
|
|
|
|
|
|
|
ثورة طبية.. ابتكار أصغر جهاز لتنظيم ضربات القلب في العالم
|
|
|
|
|
|
|
قسم شؤون المعارف ووفد من جامعة البصرة يبحثان سبل تعزيز التعاون المشترك
|
|
|