


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 6-10-2020
Date: 16-9-2019
Date: 29-5-2017
|
Friedel-Crafts Alkylation was first discovered by French scientist Charles Friedel and his partner, American scientist James Crafts, in 1877. This reaction allowed for the formation of alkyl benzenes from alkyl halides, but was plagued with unwanted supplemental activity that reduced its efficiency.
The mechanism takes place as follows:
Step one:
1.bmp?revision=1)
The first step creates a cabocation that acts as the electrophile in the reaction. This step activates the haloalkane. Secondary and teriary halides only form the free cabocation in the step.
Step two
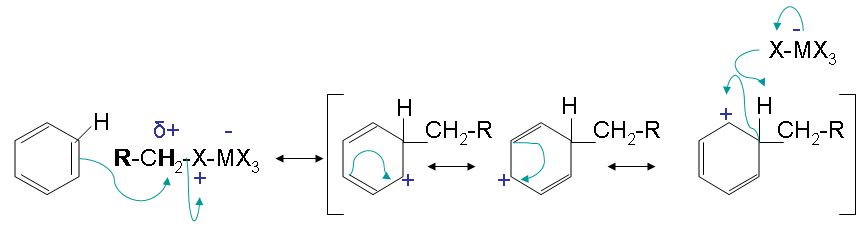
The second step has an electrophilic attack on the benzene that results in multiple resonance forms. The halogen reactions with the intermediate and picks up the hydrogen to eliminate the positive charge.
Finish
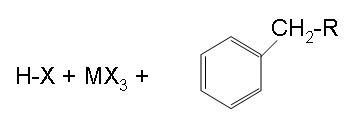
The final step shown above is the results of the end of step and shows the final products.
The reactivity of haloalkanes increases as you move up the periodic table and increase polarity. This means that an RF haloalkane is most reactive followed by RCl then RBr and finally RI. This means that the Lewis acids used as catalysts in Friedel-Crafts Alkylation reactions tend have similar halogen combinations such as BF3, SbCl5, AlCl3, SbCl5, and AlBr3, all of which are commonly used in these reactions.



|
|
|
|
تفوقت في الاختبار على الجميع.. فاكهة "خارقة" في عالم التغذية
|
|
|
|
|
|
|
أمين عام أوبك: النفط الخام والغاز الطبيعي "هبة من الله"
|
|
|
|
|
|
|
قسم شؤون المعارف ينظم دورة عن آليات عمل الفهارس الفنية للموسوعات والكتب لملاكاته
|
|
|