


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 10-12-2020
Date: 15-9-2020
Date: 6-7-2019
|
We just need to look at ethene, because what is true of C=C in ethene will be equally true of C=C in more complicated alkenes. Ethene is often modeled like this:
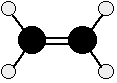
The double bond between the carbon atoms is, of course, two pairs of shared electrons. What the diagram doesn't show is that the two pairs aren't the same as each other.
One of the pairs of electrons is held on the line between the two carbon nuclei as you would expect, but the other is held in a molecular orbital above and below the plane of the molecule. A molecular orbital is a region of space within the molecule where there is a high probability of finding a particular pair of electrons.
In this diagram, the line between the two carbon atoms represents a normal bond - the pair of shared electrons lies in a molecular orbital on the line between the two nuclei where you would expect them to be. This sort of bond is called a sigma bond.
The other pair of electrons is found somewhere in the shaded part above and below the plane of the molecule. This bond is called a pi bond. The electrons in the pi bond are free to move around anywhere in this shaded region and can move freely from one half to the other.
The pi electrons are not as fully under the control of the carbon nuclei as the electrons in the sigma bond and, because they lie exposed above and below the rest of the molecule, they are relatively open to attack by other things.



|
|
|
|
5 علامات تحذيرية قد تدل على "مشكل خطير" في الكبد
|
|
|
|
|
|
|
لحماية التراث الوطني.. العتبة العباسية تعلن عن ترميم أكثر من 200 وثيقة خلال عام 2024
|
|
|