


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 11-9-2019
Date: 29-7-2018
Date: 28-11-2019
|
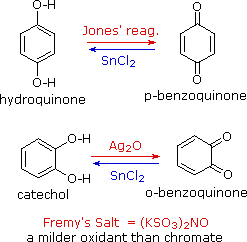
Phenols are rather easily oxidized despite the absence of a hydrogen atom on the hydroxyl bearing carbon. Among the colored products from the oxidation of phenol by chromic acid is the dicarbonyl compound para-benzoquinone (also known as 1,4-benzoquinone or simply quinone); an ortho isomer is also known. These compounds are easily reduced to their dihydroxybenzene analogs, and it is from these compounds that quinones are best prepared. Note that meta-quinones having similar structures do not exist. The redox equilibria between the dihydroxybenzenes hydroquinone and catechol and their quinone oxidation states are so facile that milder oxidants than chromate (Jones reagent) are generally preferred.
One such oxidant is Fremy's salt, shown on the right. Reducing agents other than stannous chloride (e.g. NaBH4) may be used for the reverse reaction. The position of the quinone-hydroquinone redox equilibrium is proportional to the square of the hydrogen ion concentration, as shown by the following half-reactions (electrons are colored blue). The electrode potential for this interconversion may therefore be used to measure the pH of solutions.
|
Quinone + 2H(+) |
2e(–) –2e(–) |
Hydroquinone |
|---|



|
|
|
|
علامات بسيطة في جسدك قد تنذر بمرض "قاتل"
|
|
|
|
|
|
|
أول صور ثلاثية الأبعاد للغدة الزعترية البشرية
|
|
|
|
|
|
|
مكتبة أمّ البنين النسويّة تصدر العدد 212 من مجلّة رياض الزهراء (عليها السلام)
|
|
|