
آخر المواضيع المضافة


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 5-12-2018
Date: 28-12-2018
Date: 4-3-2017
|
The molecular structure of carbonate is given below:
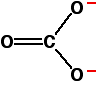
This figure shows two carbon-oxygen single bonds and one double bond, with two oxygen atoms each carrying a negative charge. However, experimental data shows that all the carbonate bonds are identical, with the charge spread out over the whole ion (concentrated on the oxygen atoms). In other words, the charges are delocalized.
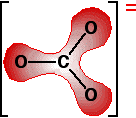
This is a more complicated version of the bonding in benzene or in ions like ethanoate. The next diagram shows the delocalized electrons. The shading shows electron density, implying a greater chance of finding electrons around the oxygen atoms than near the carbon.



|
|
|
|
التوتر والسرطان.. علماء يحذرون من "صلة خطيرة"
|
|
|
|
|
|
|
مرآة السيارة: مدى دقة عكسها للصورة الصحيحة
|
|
|
|
|
|
|
نحو شراكة وطنية متكاملة.. الأمين العام للعتبة الحسينية يبحث مع وكيل وزارة الخارجية آفاق التعاون المؤسسي
|
|
|