

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Racemic Mixtures and the Resolution of Enantiomers
المؤلف:
John McMurry
المصدر:
Organic Chemistry
الجزء والصفحة:
9th - p135
31-5-2016
7191
Racemic Mixtures and the Resolution of Enantiomers
Pasteur took an optically inactive tartaric acid salt and found that he could crystallize from it two optically active forms having what we would now call 2R,3R and 2S,3S configurations. But what was the optically inactive form he started with? It couldn’t have been meso-tartaric acid, because meso-tartaric acid is a different chemical compound and can’t interconvert with the two chiral enantiomers without breaking and re-forming chemical bonds. The answer is that Pasteur started with a 50;50 mixture of the two chiral tartaric acid enantiomers. Such a mixture is called a racemate (raa-suh-mate), or racemic mixture, and is denoted by either the symbol (-) or the prefix d,l to indicate an equal mixture of dextrorotatory and levorotatory forms. Racemates show no optical rotation because the (+) rotation from one enantiomer exactly cancels the (-) rotation from the other. Through luck, Pasteur was able to separate, or resolve, racemic tartaric acid into its (+) and (-) enantiomers.
Unfortunately, the fractional crystallization technique he used doesn’t work for most racemates, so other methods are needed. The most common method of resolution uses an acid–base reaction between the racemate of a chiral carboxylic acid (RCO2H) and an amine base (RNH2) to yield an ammonium salt:
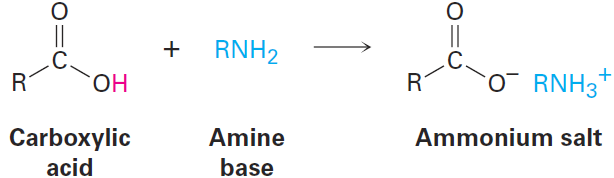
To understand how this method of resolution works, let’s see what happens when a racemic mixture of chiral acids, such as (+)- and (-)-lactic acids, reacts with an achiral amine base, such as methylamine, CH3NH2. Stereochemically, the situation is analogous to what happens when left and right hands (chiral) pick up a ball (achiral). Both left and right hands pick up the ball equally well, and the products—ball in right hand versus ball in left hand—are mirror images. In the same way, both (+)- and (-)-lactic acid react with methylamine equally well, and the product is a racemic mixture of the two enantiomers methylammonium (+)-lactate and methylammonium (-)-lactate (Figure 1-1).
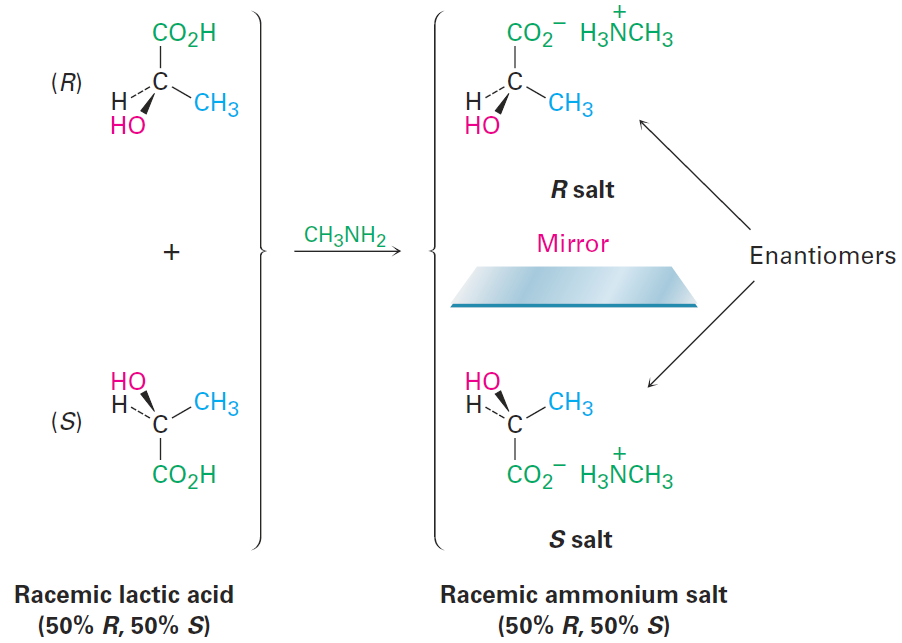
Figure 1-1 Reaction of racemic lactic acid with achiral methylamine leads to a racemic mixture of ammonium salts.
Now let’s see what happens when the racemic mixture of (+)- and (-)-lactic acids reacts with a single enantiomer of a chiral amine base, such as (R)-1-phenylethylamine. Stereochemically, the situation is analogous to when left and right hands (chiral) put on a right-handed glove (also chiral).
Left and right hands don’t put on the right-handed glove in the same way, so the products—right hand in right glove versus left hand in right glove—are not mirror images; they’re similar but different. In the same way, (+)- and (-)-lactic acids react with (R)-1-phenylethylamine to give two different products (Figure 1-2). (R)-Lactic acid reacts with (R)-1-phenylethylamine to give the R,R salt, and (S)-lactic acid reacts with the R amine to give the S,R salt. The two salts are diastereomers. They have different chemical and physical properties, and it may therefore be possible to separate them by crystallization or some other means. Once separated, acidification of the two diastereomeric salts with a strong acid allows us to isolate the two pure enantiomers of lactic acid and to recover the chiral amine for reuse.
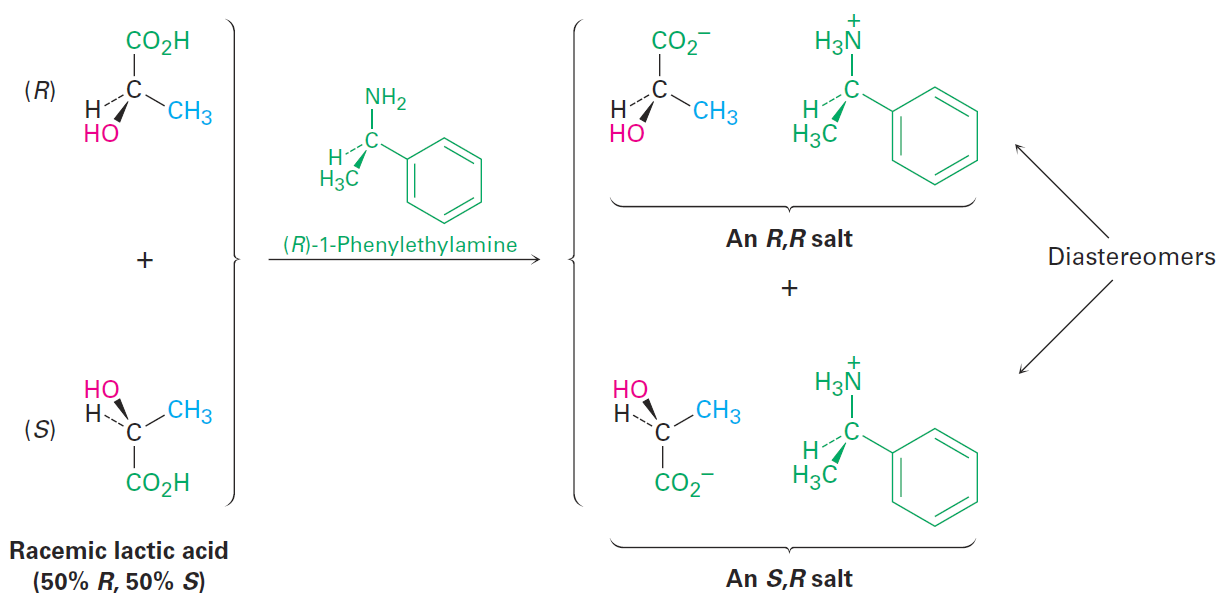
Figure 1-2 Reaction of racemic lactic acid with (R)-1-phenylethylamine yields a mixture of diastereomeric ammonium salts, which have different properties and can be separated.
 الاكثر قراءة في تجارب وتفاعلات في الكيمياء العضوية
الاكثر قراءة في تجارب وتفاعلات في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















