

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Molecular shape and the VSEPR model Valence-shell electron-pair repulsion theory
المؤلف:
CATHERINE E. HOUSECROFT AND ALAN G. SHARPE
المصدر:
INORGANIC CHEMISTRY
الجزء والصفحة:
p43
7-7-2016
6732
Molecular shape and the VSEPR model Valence-shell electron-pair repulsion theory
The shapes of molecules containing a central p-block atom tend to be controlled by the number of electrons in the valence shell of the central atom. The valence-shell electron-pair repulsion (VSEPR) theory provides a simple model for predicting the shapes of such species. The model combines original ideas of Sidgwick and Powell with extensions developed by Nyholm and Gillespie, and may be summarized as follows:
- Each valence shell electron pair of the central atom E in a molecule EXn containing E–X single bonds is stereochemically significant, and repulsions between them determine the molecular shape.
- Electron–electron repulsions decrease in the sequence: lone pair–lone pair > lone pair–bonding pair > bonding pair–bonding pair.
- Where the central atom E is involved in multiple bond formation to atoms X, electron–electron repulsions decrease in the order: triple bond–single bond > double bond – single bond > single bond–single bond.
- Repulsions between the bonding pairs in EXn depend on the difference between the electronegativities of E and X; electron – electron repulsions are less the more the E_X bonding electron density is drawn away from the central atom E.
The VSEPR theory works best for simple halides of the pblock elements, but may also be applied to species with other substituents. However, the model does not take steric factors (i.e. the relative sizes of substituents) into account. In a molecule EXn, there is a minimum energy arrangement for a given number of electron pairs. In BeCl2 (Be, group 2), repulsions between the two pairs of electrons in the valence shell of Be are minimized if the Cl_Be_Cl unit is linear. In BCl3 (B, group 13), electron–electron repulsions are minimized if a trigonal planar arrangement of electron pairs (and thus Cl atoms) is adopted. The structures in the lefthand column of Figure 1.1 represent the minimum energy structures for EXn molecules for n = 2–8 and in which there are no lone pairs of electrons associated with E. Table 1.1 gives further representations of these structures, along with their ideal bond angles. Ideal bond angles may be expected

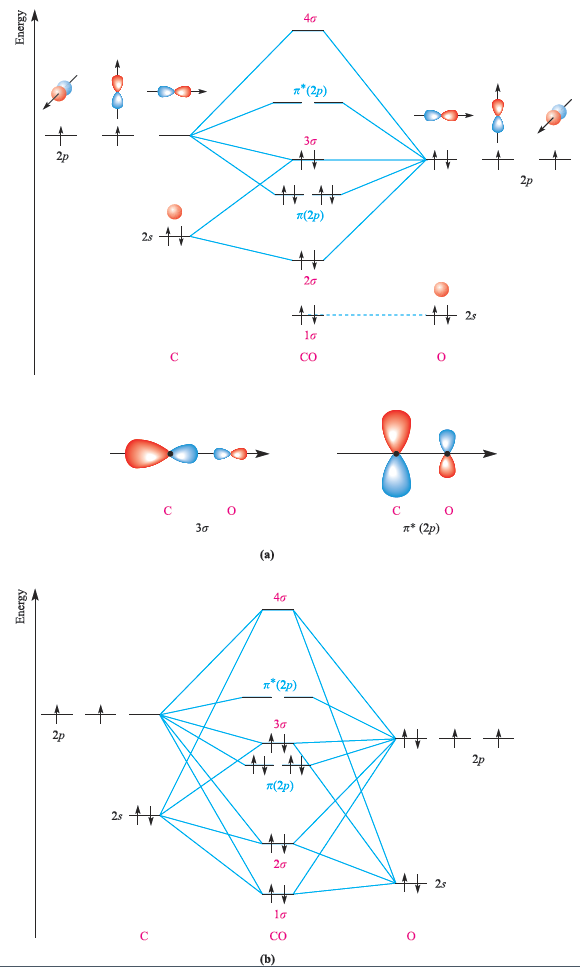
Fig. 1.1 (a) A simplified orbital interaction diagram for CO which allows for the effects of some orbital mixing. The labels 1σ, 2σ . . . rather than σ(2s) . . . are used because some orbitals contain both s and p character. (b) A more rigorous (but still qualitative) orbital interaction diagram for CO.


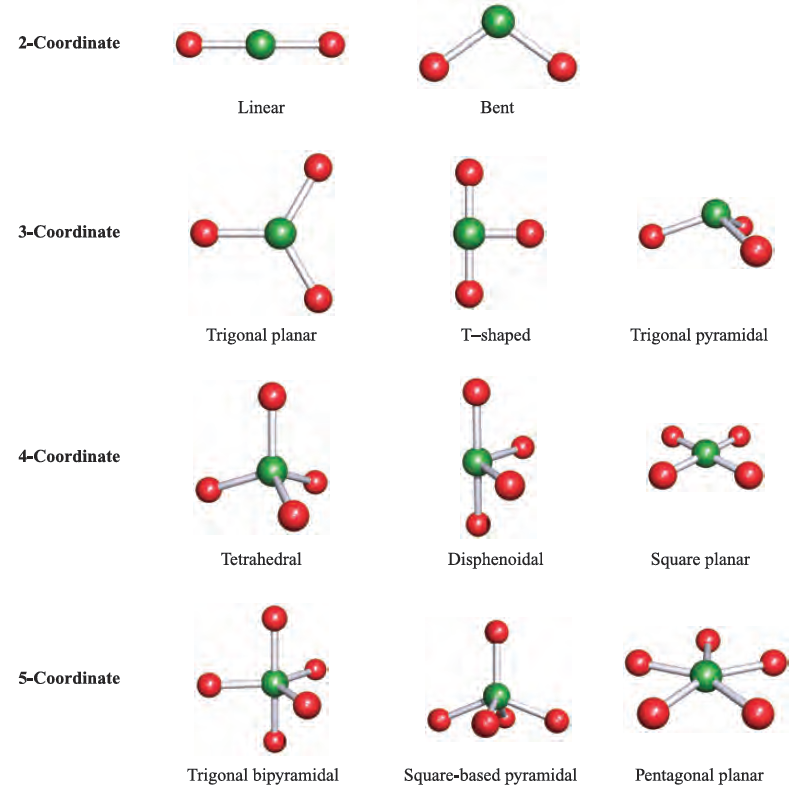
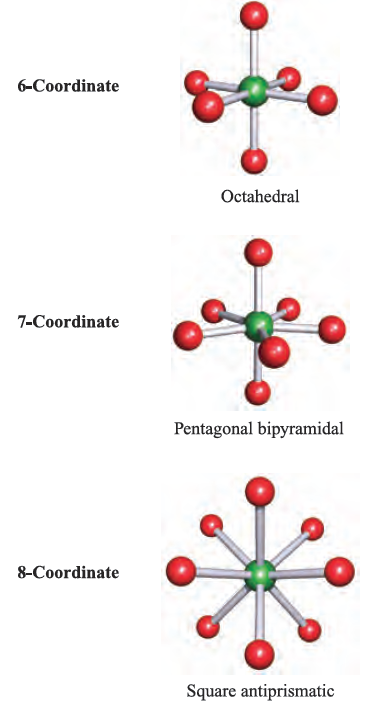
Table 1.1 ‘Parent’ shapes for EXn molecules (n = 2–8).


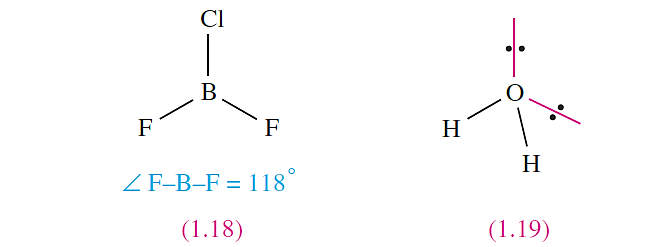
when all the X substituents are identical, but in, for example, BF2Cl (1.18) some distortion occurs because Cl is larger than F, and the shape is only approximately trigonal planar.

The presence of lone pairs is taken into account using the guidelines above and the ‘parent structures’ in Figure 1.29.
In H2O (1.19), repulsions between the two bonding pairs and two lone pairs of electrons lead to a tetrahedral arrangement but owing to the inequalities between the lone pair–lone pair, lone pair–bonding pair and bonding pair– bonding pair interactions, distortion from an ideal arrangement arises and this is consistent with the observed H_O_H bond angle of 104.58.e
 الاكثر قراءة في نظريات التآصر الكيميائي
الاكثر قراءة في نظريات التآصر الكيميائي
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















