

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Molecular orbital theory applied to the bonding in H2
المؤلف:
CATHERINE E. HOUSECROFT AND ALAN G. SHARPE
المصدر:
INORGANIC CHEMISTRY
الجزء والصفحة:
p29
14-4-2016
1968
Molecular orbital theory applied to the bonding in H2
An approximate description of the MOs in H2 can be obtained by considering them as linearcombinations of atomic orbitals (LCAOs). Each of the H atoms has one 1s atomic orbital; let the two associated wavefunctions be ψ 1 and ψ 2. The sign of the wavefunction associated with the 1s atomic orbital may be either + or -.
Just as transverse waves interfere in a constructive (inphase) or destructive (out-of-phase) manner, so too do orbitals. Mathematically, we represent the possible combinations of the two 1s atomic orbitals by equations 1.1 and 1.2, where N and N* are the normalization factors. Whereas  MO is an in-phase (bonding) interaction ψ * MO is an out-ofphase (antibonding) interaction.
MO is an in-phase (bonding) interaction ψ * MO is an out-ofphase (antibonding) interaction.

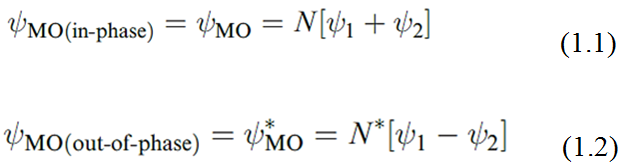
The values of N and N* are determined using equations 1.3 and 1.4 where S is the overlap integral. This is a measure of the extent to which the regions of space described by the two wavefunctions ψ 1 and ψ 2 coincide. Although we mentioned earlier that orbital interaction is efficient if the region of overlap between the two atomic orbitals is significant, the numerical value of S is still much less than unity and is often neglected giving the approximate results shown in equations 1.3 and 1.4.

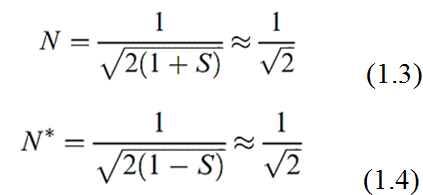
The interaction between the H 1s atomic orbitals on forming H2 may be represented by the energy level diagram in Figure 1. The bonding MO, ψ MO, is stabilized with respect to the 1s atomic orbitals, while the antibonding MO, ψ *MO, is destabilized. Each H atom contributes one electron and, by the aufbau principle, the two electrons occupy the lower of the two MOs in the H2 molecule and are spin-paired (Figure 1).

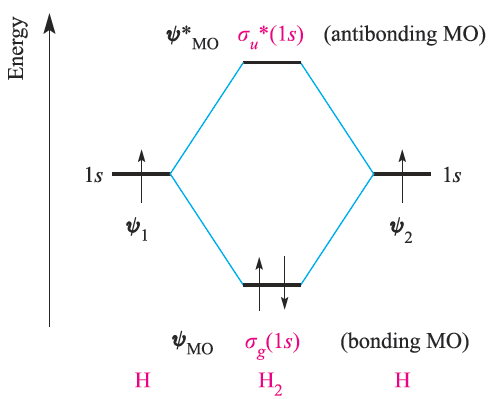
Fig. 1.1 An orbital interaction diagram for the formation of H2 from two hydrogen atoms. By the aufbau principle, the two electrons occupy the lowest energy (bonding) molecular orbital.
It is important to remember that in MO theory we construct the orbital interaction diagram first and then put in the electrons according to the aufbau principle.
 الاكثر قراءة في نظريات التآصر الكيميائي
الاكثر قراءة في نظريات التآصر الكيميائي
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















