
The temperature dependence of phase stability
 المؤلف:
Peter Atkins، Julio de Paula
المؤلف:
Peter Atkins، Julio de Paula
 المصدر:
ATKINS PHYSICAL CHEMISTRY
المصدر:
ATKINS PHYSICAL CHEMISTRY
 الجزء والصفحة:
123
الجزء والصفحة:
123
 2025-11-11
2025-11-11
 36
36
The temperature dependence of phase stability
The temperature dependence of the Gibbs energy is expressed in terms of the entropy of the system by eqn 3.50 ((∂G/∂T) p =−S). Because the chemical potential of a pure substance is just another name for its molar Gibbs energy, it follows that
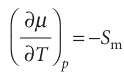
This relation shows that, as the temperature is raised, the chemical potential of a pure substance decreases: Sm > 0 for all substances, so the slope of a plot of µ against T is negative. Equation 4.1 implies that the slope of a plot of µ against temperature is steeper for gases than for liquids, because Sm(g) > Sm(l). The slope is also steeper for a liquid than the corresponding solid, because Sm(l) > Sm(s) almost always. These features are illustrated in Fig. 4.9. The steep negative slope of µ(l) results in its falling below µ(s) when the temperature is high enough, and then the liquid becomes the stable phase: the solid melts. The chemical potential of the gas phase plunges steeply downwards as the temperature is raised (because the molar entropy of the vapour is so high), and there comes a temperature at which it lies lowest. Then the gas is the stable phase and vaporization is spontaneous.
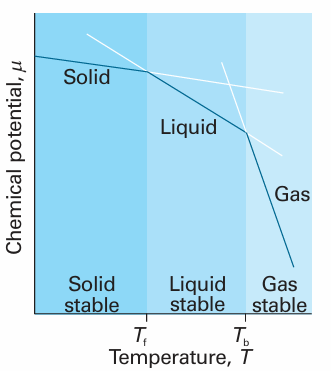
Fig. 4.9 The schematic temperature dependence of the chemical potential of the solid, liquid, and gas phases of a substance (in practice, the lines are curved). The phase with the lowest chemical potential at a specified temperature is the most stable one at that temperature. The transition temperatures, the melting and boiling temperatures (Tf and Tb, respectively), are the temperatures at which the chemical potentials of the two phases are equal.
 الاكثر قراءة في مواضيع عامة في الكيمياء الفيزيائية
الاكثر قراءة في مواضيع عامة في الكيمياء الفيزيائية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


