

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Electrocatalysis
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
الجزء والصفحة:
ص717-718
2025-10-20
54
Electrocatalysis
Key points: Overpotentials represent the kinetic barrier for electrochemical reactions and electrocatalysts may be used to increase current densities in such processes. Kinetic barriers are quite common for electrochemical reactions at the interface between a solution and an electrode and, as mentioned in Section 5.18, it is common to express these barriers as overpotentials (eta), the potential in addition to the zero-current cell potential (the emf) that must be applied to bring about an otherwise slow reaction within the cell. The overpotential is related to the current density, j (the current divided by the area of the electrode), that passes through the cell by7
i=j0ean
where j0 and a are best regarded for our purposes as empirical constants. The constant j0, the exchange current density, is a measure of the rates of the forward and reverse electrode reactions at dynamic equilibrium. For systems obeying these relations, the reaction rate (as measured by the current density) increases rapidly with increasing applied potential difference when a > 1. If the exchange current density is high, an appreciable reaction rate may be achieved with only a small overpotential. If the exchange current density is low, a high overpotential is necessary. There is therefore considerable interest in increasing the exchange current density. In an industrial process an overpotential in a synthetic step is very costly because it represents wasted energy.
A catalytic electrode surface can increase the exchange current density and hence dramatically decrease the overpotential required for sluggish electrochemical reactions, such as H2, O2, or Cl2 evolution and consumption. For example, ‘platinum black’, a finely di vided form of platinum, is very effective at increasing the exchange current density and hence decreasing the overpotential of reactions involving the consumption or evolution of H2. The role of platinum is to dissociate the strong HH bond and thereby reduce the large barrier that this bond strength imposes on reactions involving H2. Palladium also has a high exchange current density (and hence requires only a low overpotential) for H2 evolution or consumption.
The effectiveness of metals can be judged from Fig. 26.28, which also gives insight into the process. The volcano-like plot of exchange current density against MH bond enthalpy suggests that MH bond formation and cleavage are both important in the catalytic process. It appears that an intermediate MH bond energy leads to the proper balance for the existence of a catalytic cycle and the most effective metals for electrocatalysis are clustered around Group 10.
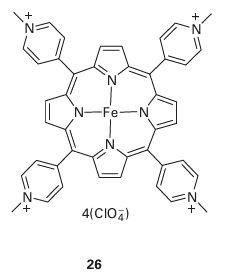
Ruthenium dioxide is an effective catalyst for both O2 and Cl2 evolution and it also is a good electrical conductor. It turns out that at high current densities RuO2 is more effective for the catalysis of Cl2 evolution than for O2 evolution. RuO2 is therefore extensively used as an electrode material in the commercial production of chorine. The electrode processes that contribute to this subtle catalytic effect do not appear to be well understood. There is great interest in devising new catalytic electrodes, particularly ones that de crease the O2 overpotential on surfaces such as graphite. Thus, tetrakis(4-N-methylpyridyl) porphyriniron (II), [Fe(TMPyP)]4+(26), has been deposited on the exposed edges of graphite electrodes (on which O2 reduction requires a high overpotential) and the resulting electrode surface was found to catalyse the electrochemical reduction of O2 . A plausible explanation for this catalysis is that [FeIII (TMPyP)] attached to the electrode (indicated below by an asterisk) is first reduced electrochemically:
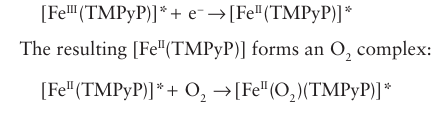
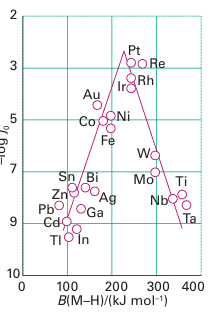
Figure 26.28 The rate of H2 evolution expressed as the logarithm of the exchange current density plotted against M H bond energy.
This iron (II) porphyrin oxygen complex then undergoes reduction to water and hydrogen peroxide:
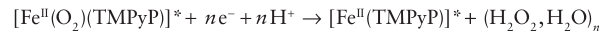
Although details of the mechanism are still elusive, this general set of reactions is in harmony with electrochemical measurements and the known properties of iron porphyrins. The investigation of porphyrin iron complexes as catalysts was motivated by Nature’s use of metalloporphyrins for oxygen activation (Section 27.10). One application where cheap and efficient electrocatalysts are much needed is in PEM fuel cells (PEM stands for ‘proton exchange membrane’ and sometimes ‘polymer electrolyte mem brane’; Box 5.1). As these systems operate at quite low temperatures, 50–100°C, they are suit able for applications that involve transport and mobile power, such as mobile telephones (cell phones). The electrolyte, a polymer that conducts protons but not electrons, separates an anode in contact with hydrogen gas and a cathode over which flows oxygen gas. As noted above hydrogen gas is readily dissociated at the anode using a platinum metal catalyst but the oxygen reduction reaction at the cathode presents more of a problem. There is a substantial overpotential associated with this reduction, which considerably reduces the efficiency of the cell, giving operating voltages near 0.7 V as compared with the theoretical value of 1.23 V. Platinum may again be used to catalyse the reaction but even so the reaction is inefficient and use of large quantities of platinum costly. Considerable research effort is being applied to finding better electro- catalysts and recently the alloy Pt3 Ni (111 surface) has proved to have greatly enhanced properties.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















