

 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 2-5-2017
Date: 17-4-2017
Date: 19-3-2016
|
Distribution of Hydrophobic Ionogenic Organic Compounds
Some highly hydrophobic weak acids and bases exhibit substantial hydrophobicity even in the ionized state. For highly hydrophobic ionogenic organic compounds, not only is transfer of the neutral species between the aqueous phase and the immiscible phase important, but the transfer of the hydrophobic, ionized, organic species as free ions or ion pairs may also be significant. Mathematically, this is described by refining then-octanol/ water partition coefficient to reflect the pHdependent distribution between water (W) andn octanol (O) of chemical X in both the ionized and nonionized forms. If chemical X is a weak acid, HA, the distribution ratio is
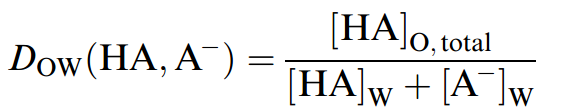
where [HA]O;total is the sum of all neutral species, free ions, and ions paired with inorganic counterions that transfer to octanol.
For example, the ratio of then-octanol/water distribution coefficient of the nondissociated species to that of the ionic species is nearly 10,000 for 3-methyl-2-nitrophenol, but only about 1000 for pentachlorophenol because of the greater significance of the hydrophobicity of the ionized form of pentachlorophenol. The logarithm of then-octanol/water distribution coefficient of pentachlorophenol as the phenolate is about 2 (determined at pH 12, and 0.1MKCl), which indicates significant distribution of the ionized form into then-octanol phase. Extraction of such highly hydrophobic ionogenic organic compounds can result from mixed-mode mechanisms that incorporate both the hydrophobic and ionic character of the compound.



|
|
|
|
دخلت غرفة فنسيت ماذا تريد من داخلها.. خبير يفسر الحالة
|
|
|
|
|
|
|
ثورة طبية.. ابتكار أصغر جهاز لتنظيم ضربات القلب في العالم
|
|
|
|
|
|
|
العتبة العباسية المقدسة تستعد لإطلاق الحفل المركزي لتخرج طلبة الجامعات العراقية
|
|
|