


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 1-1-2018
Date: 24-12-2018
Date: 26-12-2018
|
The chloralkali industry
The chloralkali industry produces huge quantities of NaOH and Cl2 by the electrolysis of aqueous NaCl (brine).
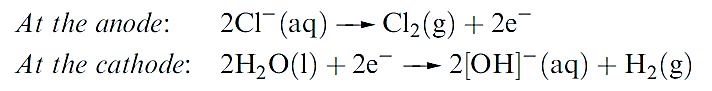
Three types of electrolysis cell are available:
Aqueous NaOH from the electrolytic process is evaporated to give solid NaOH (caustic soda) as a white, translucent solid which is fused and cast into sticks, or made into flakes or pellets. Uses of NaOH are summarized.
The chloralkali industry illustrates an interesting market problem. While the electrolysis of brine produces NaOH and Cl2 in a fixed molar ratio, the markets for the two chemicals are different and unrelated. Interestingly, prices of the two chemicals follow opposite trends; in times of recession, demand for Cl2 falls more sharply than that of NaOH, with the result that the price of Cl2 falls as stocks build up. Conversely, industrial demand for Cl2 increases faster than that of NaOH when the economy is strong; consequently, the price of the alkali falls as stocks increase. The net result is clearly important to the long-term stability of the chloralkali industry as a whole.



|
|
|
|
التوتر والسرطان.. علماء يحذرون من "صلة خطيرة"
|
|
|
|
|
|
|
مرآة السيارة: مدى دقة عكسها للصورة الصحيحة
|
|
|
|
|
|
|
نحو شراكة وطنية متكاملة.. الأمين العام للعتبة الحسينية يبحث مع وكيل وزارة الخارجية آفاق التعاون المؤسسي
|
|
|