


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 9-5-2016
Date: 13-8-2017
Date: 11-9-2017
|
Free Radical Polymerization
Free radical initiators can polymerize olefinic compounds. These chemical compounds have a weak covalent bond that breaks easily into two free radicals when subjected to heat. Peroxides, hydroperoxides and azo compounds are commonly used. For example, heating peroxybenzoic acid forms two free radicals, which can initiate the polymerization reaction:
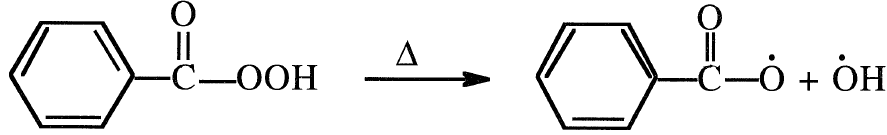
Free radicals are highly reactive, short lived, and therefore not selective.
Chain transfer reactions often occur and result in a highly branched product polymer. For example, the polymerization of ethylene using an organic peroxide initiator produces highly branched polyethylene. The branches result from the abstraction of a hydrogen atom by a propagating polymer intermediate, which creates a new active center. The new center can add more ethylene molecules, forming a long branch:
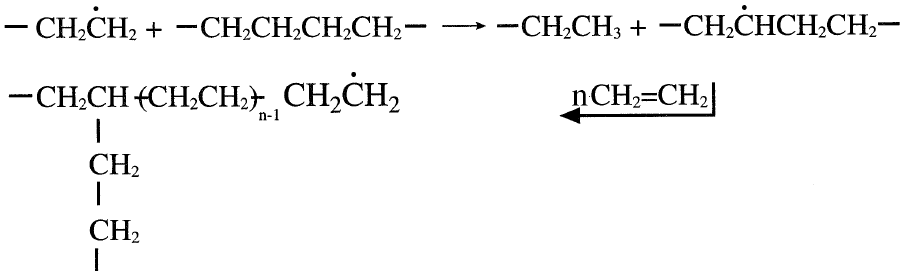
Intermolecular chain transfer reactions may occur between two propagating polymer chains and result in the termination of one of the chains.
Alternatively, these reactions take place by an intramolecular reaction by the coiling of a long chain. Intramolecular chain transfer normally results in short branches:
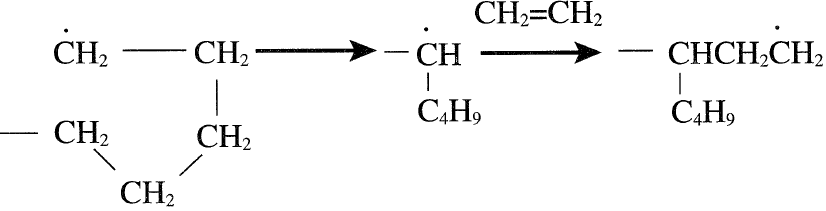
Free radical polymers may terminate when two propagating chains combine. In this case, the tail-to-tail addition mode is most likely. Polymer propagation stops with the addition of a chain transfer agent. For example, carbon tetrachloride can serve as a chain transfer agent:

The ˙CCl3 free radical formed can initiate a new polymerization reaction.



|
|
|
|
التوتر والسرطان.. علماء يحذرون من "صلة خطيرة"
|
|
|
|
|
|
|
مرآة السيارة: مدى دقة عكسها للصورة الصحيحة
|
|
|
|
|
|
|
نحو شراكة وطنية متكاملة.. الأمين العام للعتبة الحسينية يبحث مع وكيل وزارة الخارجية آفاق التعاون المؤسسي
|
|
|