


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 18-9-2017
Date: 23-11-2015
Date: 11-9-2017
|
CONDENSATION POLYMERIZATION
(Step-Reaction Polymerization)
Though less prevalent than addition polymerization, condensation polymerization produces important polymers such as polyesters, polyamides (nylons), polycarbonates, polyurethanes, and phenolformaldehyde resins.
In general, condensation polymerization refers to
1. A reaction between two different monomers. Each monomer possesses at least two similar functional groups that can react with the functional groups of the other monomer. For example, a reaction of a diacid and a dialcohol (diol) can produce polyesters:

A similar reaction between a diamine and a diacid can also produce polyamides.
2. Reactions between one monomer species with two different functional groups. One functional group of one molecule reacts with the other functional group of the second molecule. For example, polymerization of an amino acid starts with condensation of two monomer molecules:
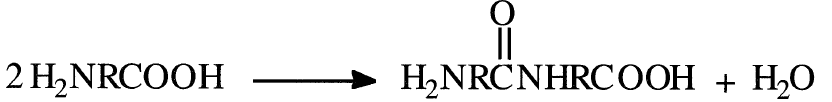
In these two examples, a small molecule (water) results from the condensation reaction. Ring opening polymerization of lactams can also be considered a condensation reaction, although a small molecule is not eliminated. This type is noted later in this chapter under “Ring Opening Polymerization.”
Condensation polymerization is also known as step-reaction polymerization because the reactions occur in steps. First, a dimer forms, then a trimer, next a tetramer, and so on until the polymer terminates. Although step polymerizations are generally slower than addition polymerizations, with long reaction times required for high conversions, the monomers disappear fast. The reaction medium contains only dimers, trimers, tetramers, and so on. For example, the dimer formed from the condensation of a diacid and a diol (reaction previously shown) has hydroxyl and carboxyl endings that can react with either a diacid or a diol to form a trimer:

The compounds formed continue condensation as long as the species present have different endings. The polymer terminates by having one of the monomers in excess. This produces a polymer with similar endings. For example, a polyester formed with excess diol could be represented:
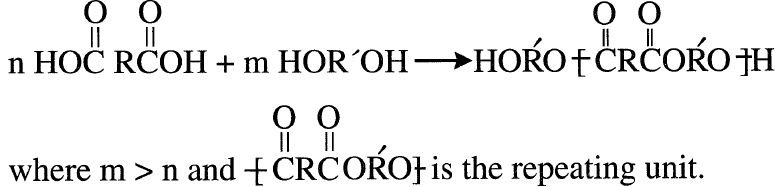
In these reactions, the monomers have two functional groups (whether one or two monomers are used), and a linear polymer results. With more than two functional groups present, crosslinking occurs and a thermosetting polymer results. Example of this type are polyurethanes and urea formaldehyde resins.
Acid catalysts, such as metal oxides and sulfonic acids, generally catalyze condensation polymerizations. However, some condensation polymers form under alkaline conditions. For example, the reaction of formaldehyde with phenol under alkaline conditions produces methylolphenols, which further condense to a thermosetting polymer.



|
|
|
|
التوتر والسرطان.. علماء يحذرون من "صلة خطيرة"
|
|
|
|
|
|
|
مرآة السيارة: مدى دقة عكسها للصورة الصحيحة
|
|
|
|
|
|
|
نحو شراكة وطنية متكاملة.. الأمين العام للعتبة الحسينية يبحث مع وكيل وزارة الخارجية آفاق التعاون المؤسسي
|
|
|