


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 13-8-2017
Date: 30-7-2017
Date: 10-8-2017
|
Hydrocracking Catalysts and Reactions
The dual-function catalysts used in hydrocracking provide high surface area cracking sites and hydrogenation-dehydrogenation sites. Amorphous silica-alumina, zeolites, or a mixture of them promote carbonium ion formation. Catalysts with strong acidic activity promote isomerization, leading to a high iso/normal ratios.30 The hydrogenation dehydrogenation activity, on the other hand, is provided by catalysts such as cobalt, molybdenum, tungsten, vanadium, palladium, or rare earth elements. As with catalytic cracking, the main reactions occur by carbonium ion and beta scission, yielding two fragments that could be hydrogenated on the catalyst surface. The main hydro-cracking reaction could be illustrated by the first-step formation of a carbocation over the catalyst surface:
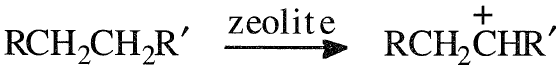
The carbocation may rearrange, eliminate a proton to produce an olefin, or crack at a beta position to yield an olefin and a new carbocation. Under an atmosphere of hydrogen and in the presence of a catalyst with hydrogenation-dehydrogenation activity, the olefins are hydrogenated to paraffinic compounds. This reaction sequence could be represented as follows:
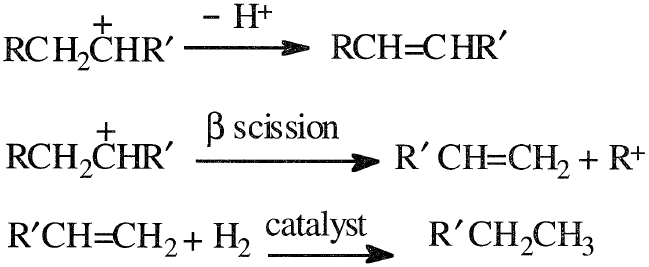
As can be anticipated, most products from hydrocracking are saturated. For this reason, gasolines from hydrocracking units have lower octane ratings than those produced by catalytic cracking units; they have a lower aromatic content due to high hydrogenation activity. Products from hydrocracking units are suitable for jet fuel use. Hydrocracking also produces light hydrocarbon gases (LPG) suitable as petrochemical feedstocks. Other reactions that occur during hydrocracking are the fragmentation followed by hydrogenation (hydrogenolysis) of the complex asphaltenes and heterocyclic compounds normally present in the feeds.
Dealkylation, fragmentation, and hydrogenation of substituted polynuclear aromatics may also occur. The following is a representative example of hydrocracking of a substituted anthracene.
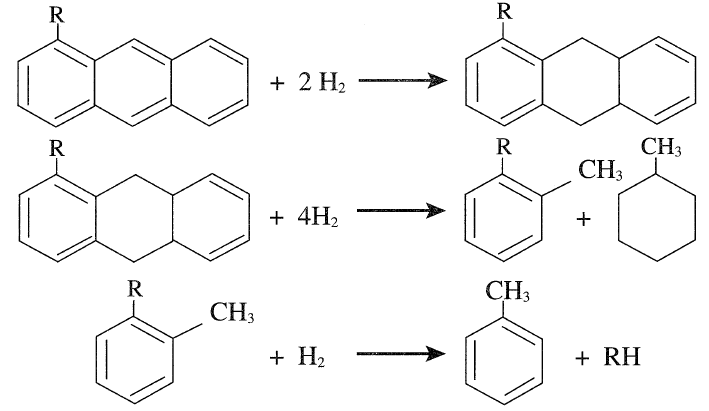
It should be noted, however, that this reaction sequence may be different from what may actually be occurring in the reactor. The reactions proceed at different rates depending on the process variables. Hydrodesulfurization of complex sulfur compounds such as dibenzothiophene also occurs under these conditions. The desulfurized product may crack to give two benzene molecules:
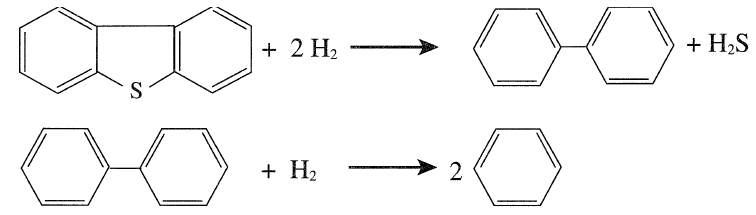



|
|
|
|
التوتر والسرطان.. علماء يحذرون من "صلة خطيرة"
|
|
|
|
|
|
|
مرآة السيارة: مدى دقة عكسها للصورة الصحيحة
|
|
|
|
|
|
|
نحو شراكة وطنية متكاملة.. الأمين العام للعتبة الحسينية يبحث مع وكيل وزارة الخارجية آفاق التعاون المؤسسي
|
|
|