


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 3-5-2017
Date: 25-2-2018
Date: 25-2-2018
|
Interconversion of Composition Measures
Given the solution composition in mass percent, it is easy to compute the mole fractions of solute and solvent and the molal concentration of solute.
Example 1
What are the mole fractions and molal concentrations in a 10% by weight solution of CH3OH in water? We first compute the number of moles of CH3OH and water:
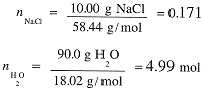
The total number of moles is 5.33 and the mole fractions are:
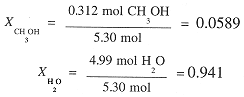
The molal concentrations of CH3OH is computed from the number of moles and the mass of H2O.
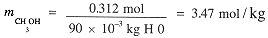
Molar concentrations involve the volume of the solution and to get this from the mass, we need the density.
Example 2
Given that the density of 10.0% aqueous NaCl is 1.071 g/cm3, what is the molar concentrations of Na+ and Cl-?
Two approaches are possible. (1) We know how many moles of NaCl are contained in 100 g of solution. Thus we have
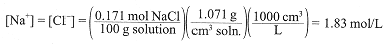
(2) One liter of solution weighs 1071 g and contains 107.1 g NaCl/L; converting to moles, we have [NaCl] = [Na+] = [Cl-] = 1.83 mol/



|
|
|
|
التوتر والسرطان.. علماء يحذرون من "صلة خطيرة"
|
|
|
|
|
|
|
مرآة السيارة: مدى دقة عكسها للصورة الصحيحة
|
|
|
|
|
|
|
نحو شراكة وطنية متكاملة.. الأمين العام للعتبة الحسينية يبحث مع وكيل وزارة الخارجية آفاق التعاون المؤسسي
|
|
|