


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 24-6-2017
Date: 19-3-2016
Date: 24-6-2017
|
Relative Atomic Masses
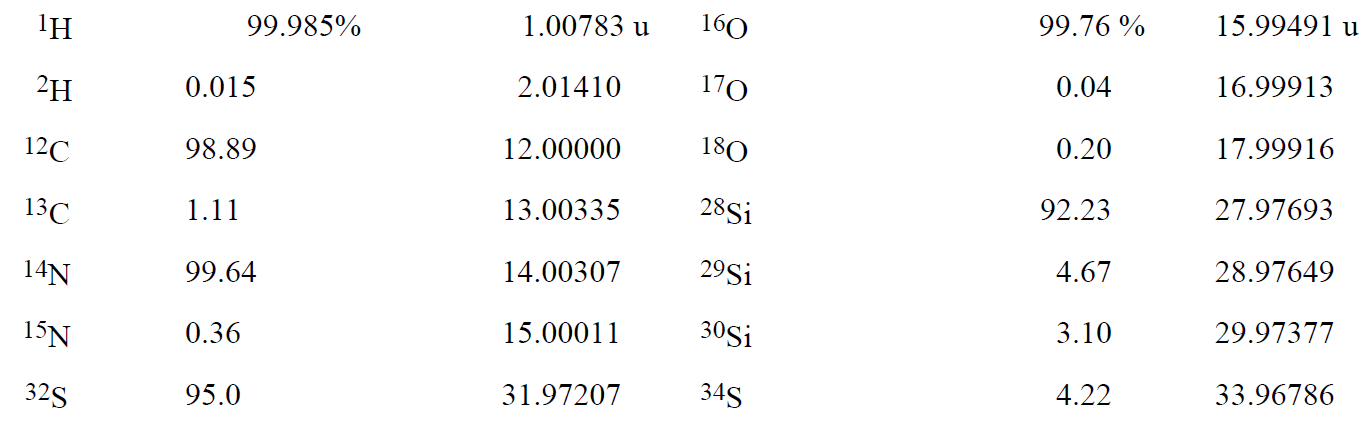
Because the mass of an atom is very small, it is convenient to define a special unit that avoids large negative exponents. This unit, called the atomic mass unit and designated by the symbol u (some authors use the abbreviation amu), is defined as exactly 1/12 the mass of a 12C atom. Thus the mass of a 12C atom is exactly 12 u. The masses and abundances of some other nuclides are listed in Table 1-1.
Naturally occurring silicon is 92.23% 28Si, 4.67% 29Si, and 3.10% 30Si. For chemical purposes, it is sufficient to know the average mass of a silicon atom in this isotopic mixture. These average masses are designated by Ar (E), where E is the symbol for the particular element. For example, the average mass of silicon atoms is
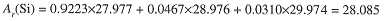
The term atomic mass will be understood to mean average atomic mass; nuclidic mass refers to one particular isotope of an element. Atomic masses are used in nearly all chemical calculations.
Table 1-1. Some Nuclidic Masses in Atomic Mass Units



|
|
|
|
دخلت غرفة فنسيت ماذا تريد من داخلها.. خبير يفسر الحالة
|
|
|
|
|
|
|
ثورة طبية.. ابتكار أصغر جهاز لتنظيم ضربات القلب في العالم
|
|
|
|
|
|
|
قسم شؤون المعارف ووفد من جامعة البصرة يبحثان سبل تعزيز التعاون المشترك
|
|
|