


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية | Evidence for the Mechanism of Electrophilic dditions: Carbocation Rearrangements |
|
|
|
Read More
Date: 12-7-2019
Date: 19-7-2019
Date: 20-1-2020
|
Evidence for the Mechanism of Electrophilic dditions: Carbocation Rearrangements
How do we know that the carbocation mechanism for electrophilic addition reactions of alkenes is correct? The answer is that we don’t know it’s correct; at least we don’t know with complete certainty. Although an incorrect reaction mechanism can be disproved by demonstrating that it doesn’t account for observed data, a correct reaction mechanism can never be entirely proven.
The best we can do is to show that a proposed mechanism is consistent with all known facts. If enough facts are accounted for, the mechanism is probably correct.
One of the best pieces of evidence supporting the carbocation mechanism for the electrophilic addition reaction was discovered during the 1930s by F. C. Whitmore of Pennsylvania State University, who found that structural rearrangements often occur during the reaction of HX with an alkene.
For example, reaction of HCl with 3-methyl-1-butene yields a substantial amount of 2-chloro-2-methylbutane in addition to the “expected” product, 2-chloro-3-methylbutane.
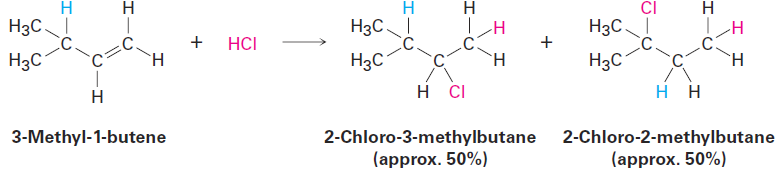
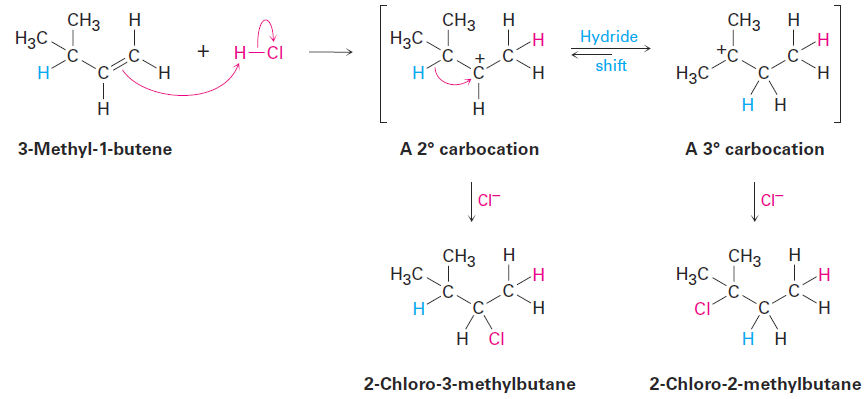
If the reaction takes place in a single step, it would be difficult to account for rearrangement, but if the reaction takes place in several steps, rearrangement is more easily explained. Whitmore suggested that it is a carbocation intermediate that undergoes rearrangement. The secondary carbocation intermediate formed by protonation of 3-methyl-1-butene rearranges to a more stable tertiary carbocation by a hydride shift—the shift of a hydrogen atom and its electron pair (a hydride ion, :H-) between neighboring carbons.
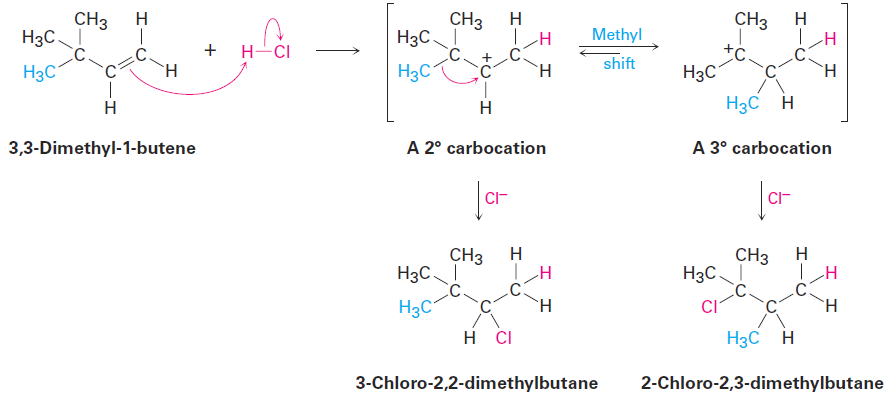
Carbocation rearrangements can also occur by the shift of an alkyl group with its electron pair. For example, reaction of 3,3-dimethyl-1-butene with HCl leads to an equal mixture of unrearranged 3-chloro-2,2-dimethylbutane and rearranged 2-chloro-2,3-dimethylbutane. In this instance, a secondary carbocation rearranges to a more stable tertiary carbocation by the shift of a methyl group.
Note the similarities between the two carbocation rearrangements: in both cases, a group (:H- or :CH3-) moves to an adjacent positively charged carbon, taking its bonding electron pair with it. Also in both cases, a less stable carbocation rearranges to a more stable ion. Rearrangements of this kind are a common feature of carbocation chemistry and are particularly important in the biological pathways by which steroids and related substances are synthesized.
An example is the following hydride shift that occurs during the biosynthesis of cholesterol.
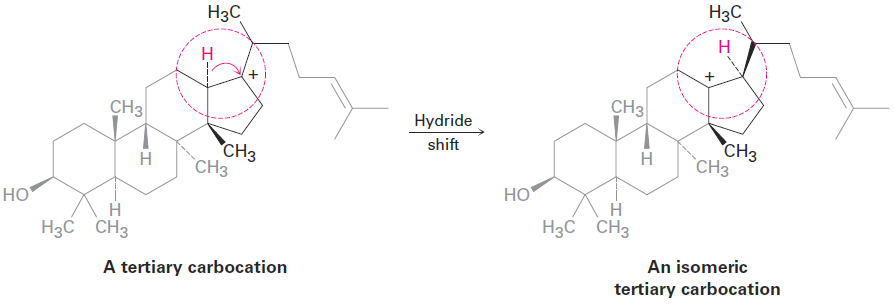
A word of advice that we’ve noted before and will repeat on occasion: biological molecules are often larger and more complex in appearance than the molecules chemists work with in the laboratory, but don’t be intimidated. When looking at any chemical transformation, whether biochemical or not, focus on the part of the molecule where the change is occurring and don’t worry about the rest. The tertiary carbocation just pictured looks complicated, but all the chemistry is taking place in the small part of the molecule inside the red circle.



|
|
|
|
التوتر والسرطان.. علماء يحذرون من "صلة خطيرة"
|
|
|
|
|
|
|
مرآة السيارة: مدى دقة عكسها للصورة الصحيحة
|
|
|
|
|
|
|
نحو شراكة وطنية متكاملة.. الأمين العام للعتبة الحسينية يبحث مع وكيل وزارة الخارجية آفاق التعاون المؤسسي
|
|
|