


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 22-2-2017
Date: 14-9-2016
Date: 27-2-2017
|
Coordination number 5
The limiting structures for 5-coordination are the trigonal bipyramid and square-based pyramid. In practice, many structures lie between these two extremes, and we have already emphasized that the energy differences between trigonal bipyramidal and square-based pyramidal structures are often small. Among simple 5-coordinate complexes are trigonal bipyramidal [CdCl5]3-, [HgCl5]3- and [CuCl5]3- (d10) and a series of square-based pyramidal oxo- or nitrido-complexes in which the oxo or nitrido ligand occupies the axial site:
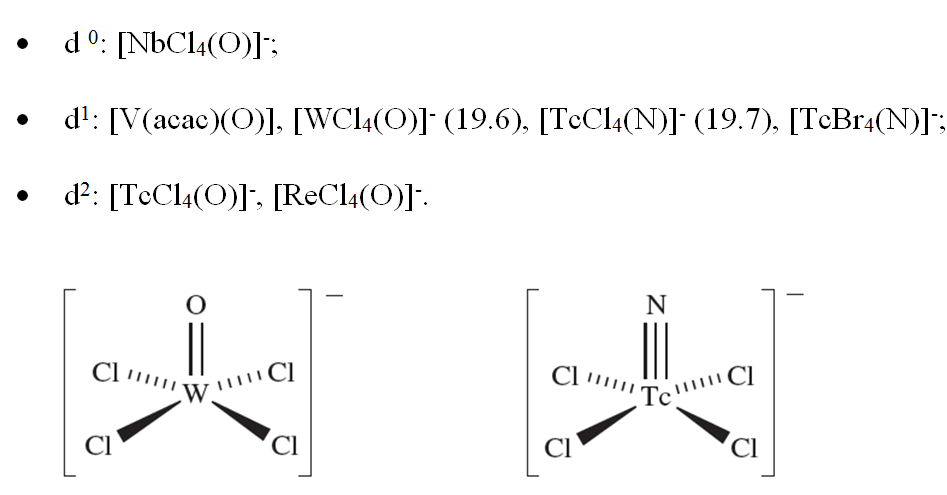
(19.6) (19.7)
The formulae of some complexes may misleadingly suggest ‘5-coordinate’ metal centres: e.g. Cs3CoCl5 is actually Cs3[CoCl4]Cl. 5-Coordinate structures are found for many compounds with polydentate amine, phosphine or arsine ligands. Of particular interest among these are complexes containing tripodal ligands (19.3) in which the central atom is a donor atom; this makes the ligand ideally suited to occupy one axial and the three equatorial sites of a trigonal bipyramidal complex as in [CoBr{N(CH2CH2NMe2)3}], [Rh(SH){P(CH2CH2PPh2)3}] and [Zn{N(CH2CH2NH2)3}Cl]+ (Figure 19.5b). On the other hand, the conformational constraints of the ligands may result in a preference for a square-based pyramidal complex in the solid state, e.g. [Cu(bpy){NH(CH2CO2)2}].6H2O (Figure 19.5c).



|
|
|
|
لخفض ضغط الدم.. دراسة تحدد "تمارين مهمة"
|
|
|
|
|
|
|
طال انتظارها.. ميزة جديدة من "واتساب" تعزز الخصوصية
|
|
|
|
|
|
|
انطلاق فعالية المسير للمشاركين بحفل التخرج المركزي لطلبة الجامعات العراقية عند المرقدين الطاهرين
|
|
|