


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 16-2-2018
Date: 14-5-2020
Date: 1-1-2017
|
The reactivity of the metals
In general, the metals are moderately reactive and combine to give binary compounds when heated with dioxygen, sulfur or the halogens (e.g. reactions 1.1–1.3), product stoichiometry depending, in part, on the available oxidation states (see below).
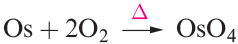 (1.1)
(1.1)
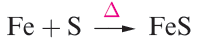 (1.2)
(1.2)
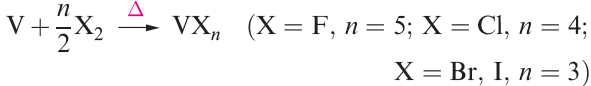 (1.3)
(1.3)
Most d-block metals should, on thermodynamic grounds liberate H2 from acids but, in practice, many do not since they are passivated by a thin surface coating of oxide or by having a high dihydrogen overpotential, or both. Silver, gold and mercury (i.e. late, second and third row metals) are, even in the thermodynamic sense, the least reactive metals known. For example, gold is not oxidized by atmospheric O2 or attacked by acids, except by a 3 :1 mixture of concentrated HCl and HNO3 (aqua regia).



|
|
|
|
التوتر والسرطان.. علماء يحذرون من "صلة خطيرة"
|
|
|
|
|
|
|
مرآة السيارة: مدى دقة عكسها للصورة الصحيحة
|
|
|
|
|
|
|
نحو شراكة وطنية متكاملة.. الأمين العام للعتبة الحسينية يبحث مع وكيل وزارة الخارجية آفاق التعاون المؤسسي
|
|
|