


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 24-1-2018
Date: 27-4-2019
Date: 24-1-2018
|
Predicting the type of bond
Electronegativities are useful because they give information about what will happen to the bonding pair of electrons when two atoms bond.
A bond in which the electron pair is equally shared is called a nonpolar covalent bond. You have a nonpolar covalent bond anytime the two atoms involved in the bond are the same or anytime the difference in the electronegativities of the atoms involved in the bond is very small. For example, consider the Cl2 molecule. The table in Figure below shows that chlorine has an electronegativity value of 3.0. Each chlorine atom attracts the bonding electrons with a force of 3.0. Because there’s an equal attraction, the bonding electron pair is shared equally between the two chlorine atoms and is located halfway between the two atoms.
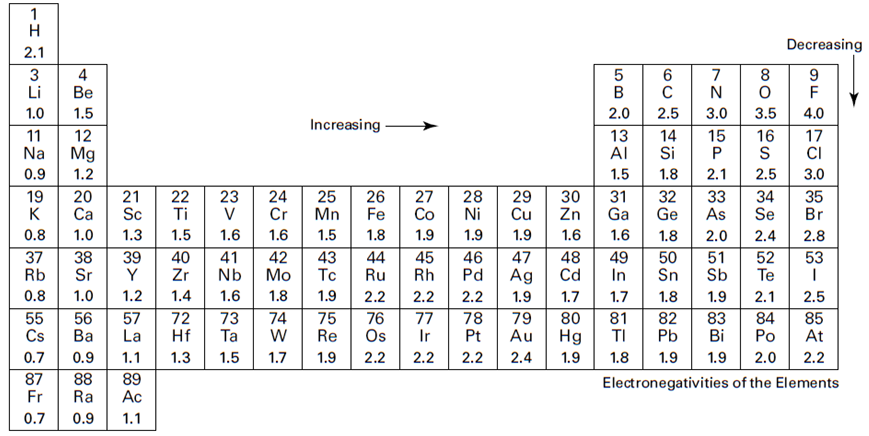
A bond in which the electron pair is shifted toward one atom is called a polar covalent bond. The atom that more strongly attracts the bonding electron pair is slightly more negative, and the other atom is slightly more positive. The larger the difference in the electronegativities, the more negative and positive the atoms become. Consider hydrogen chloride (HCl). Hydrogen has an electronegativity of 2.1, and chlorine has an electronegativity of 3.0. Because chlorine has a larger electronegativity value, the electron pair that’s bonding HCl together shifts toward the chlorine atom.
If the two atoms have extremely different electronegativities, the atoms will probably form ionic, not covalent bonds. For instance, sodium chloride (NaCl) is ionically bonded. An electron has transferred from sodium to chlorine. Sodium has an electronegativity of 1.0, and chlorine has an electronegativity of 3.0. That’s an electronegativity difference of 2.0 (3.0 – 1.0), making the bond between the two atoms very, very polar.
The electronegativity difference provides another way of predicting which kind of bond will form between two elements. Here are some guidelines on whether a bond will be polar or nonpolar, covalent or ionic:

The presence of a polar covalent bond in a molecule can have some pretty dramatic effects on the properties of a molecule, as you see in the next section.



|
|
|
|
للعاملين في الليل.. حيلة صحية تجنبكم خطر هذا النوع من العمل
|
|
|
|
|
|
|
"ناسا" تحتفي برائد الفضاء السوفياتي يوري غاغارين
|
|
|
|
|
|
|
نحو شراكة وطنية متكاملة.. الأمين العام للعتبة الحسينية يبحث مع وكيل وزارة الخارجية آفاق التعاون المؤسسي
|
|
|