


 الفيزياء الكلاسيكية
الفيزياء الكلاسيكية
 الكهربائية والمغناطيسية
الكهربائية والمغناطيسية
 علم البصريات
علم البصريات
 الفيزياء الحديثة
الفيزياء الحديثة
 النظرية النسبية
النظرية النسبية
 الفيزياء النووية
الفيزياء النووية
 فيزياء الحالة الصلبة
فيزياء الحالة الصلبة
 الليزر
الليزر
 علم الفلك
علم الفلك
 المجموعة الشمسية
المجموعة الشمسية
 الطاقة البديلة
الطاقة البديلة
 الفيزياء والعلوم الأخرى
الفيزياء والعلوم الأخرى
 مواضيع عامة في الفيزياء
مواضيع عامة في الفيزياء|
Read More
Date: 11-4-2016
Date: 24-1-2021
Date: 31-1-2021
|
CREATING AN INVERSION
Consider now a situation where energy is injected into a system at thermal equilibrium to cause a population of atoms at a higher energy level to be greater than that of a lower level. Such a non-equilibrium condition is indeed required for lasing action in almost all lasers.1 Energy may be injected selectively to pump an upper energy level from which transitions occur to a lower level (in doing so, photons of light are emitted). It is not necessary to excite all upper energy levels in the gas: The requirement is that a higher population of atoms exists at the upper level of a particular transition than at the lower level of that same transition. The reasons for this will become clear when considering rates of stimulated emission.
Pumping is the process of supplying energy to the laser medium to excite the upper energy levels. It may be accomplished by any number of means, including electrical, thermal, optical, chemical, or nuclear. Most common lasers utilize electrical pumping (as seen in the previous example with the helium–neon laser) or optical pumping, as shown in Figure 1.1. In the case of optical pumping, light from a flash lamp or an arc lamp is focused onto a rod containing the lasing atoms. Common laser rods include ruby (chromium ions in an aluminum oxide host glass) and YAG (neodymium ions in a yttrium aluminum garnet host glass). The lasing atoms (chromium or neodymium in this case) absorb photons of incident pump light and

Figure 1.1. Optical pumping for lasers.
become excited to upper energy levels. An electrically pumped laser (like most gas lasers) uses an electrical discharge to excite atoms directly to high energy levels. In some lasers (e.g., the He Ne), the actual lasing atom is neon, which is pumped with energy via a multistep process involving the transfer of energy between two atomic species. In this case, helium serves to pump the neon atoms when a helium atom (excited by the electrical discharge) collides with a neon atom, transferring energy to the neon atom.
In addition to optical and electrical pumping methods (the most common), other ways of injecting energy into a laser include chemical reaction and nuclear pumping. Regardless of the method, the end goal of pumping is to excite high-energy states within the lasing medium so that the population of atoms at a high-energy state is greater than the population of atoms at a low-energy state for the lasing transition. One might now wonder how pumping can be effected to ensure that the upper level is filled without filling the lower level (i.e., creating an inversion). The answer lies in the design of the pumping source or method to ensure that the upper level is pumped selectively while the lower level is not. Consider the He Ne gas laser, which consists of a glass tube containing both helium and neon gases in which an electrical discharge occurs. The discharge, similar to that in the tubes of a neon sign, excites helium atoms by electron collision. The vast majority of energy is wasted in the process, some of it in the pink glow given off by the tube, still more in the form of heat. Some excited helium atoms (with a specific energy of 20.61 eV) can collide with neon atoms and in doing so transfer energy to them at an almost identical energy level (the target is the neon level at 20.66 eV). The energy levels involved are depicted in Figure 1.2. This energy level for neon, which accepts energy from the helium, is the upper level for the transitions that produce the visible He Ne laser output. As expected for a multi electron atom such as neon, quantum mechanics predicts a splitting of energy levels into a series of closely spaced levels. In the case of neon, the actual upper level at 20.66 eV splits into four discrete levels.

Figure 1.2. Pumping mechanism in the helium–neon laser.
Through this process neon atoms are then pumped, quite selectively, to an upper energy level from which lasing transitions can occur (helium lacks a level close to the lower lasing level of neon, so it does not receive energy from collisions with helium). This scheme ensures that the upper energy level for the transition, which produces laser light, has a higher population of neon atoms than the lower level. We can contrast the situation in the He Ne laser to the Boltzmann distribution of neon at thermal equilibrium in Figure 1.3 to see the inversion of the levels.
In Figure 1.3 the populations of neon atoms are compared both at thermal equilibrium and in an operating laser. The two levels of interest are the 2p5 5s1 and 2p53p1 levels. (This is the configuration of the excited neon atom, which describes the state of electrons in the atom. Recall that neon at ground state has a configuration 2p6 or, more fully expanded, 1s2 2s2 2p6.) These are the upper and lower lasing levels for the 632.8-nm red transition, the common red He Ne laser. Numerous other lasing transitions are possible
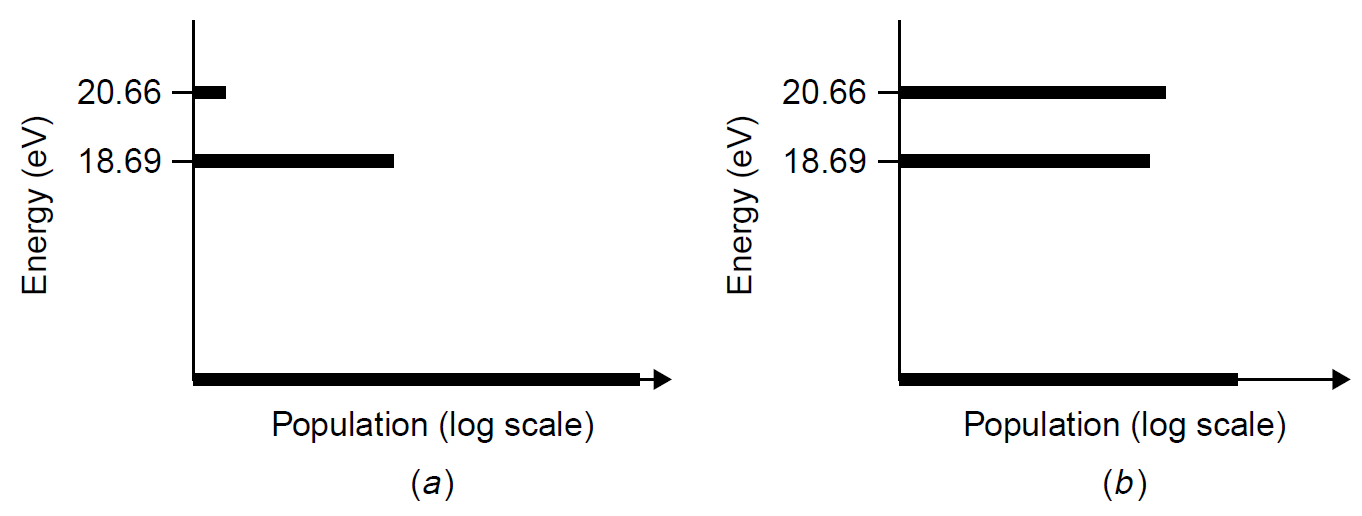
Figure 1.3. Population energies of neon atoms (a) at equilibrium and (b) in a He Ne laser.
given neon’s many energy levels (e.g., electron spin leads to a hyperfine structure where the 2p5 3p1 level is actually 10 closely spaced levels, giving us 10 possible laser transitions!). Using Boltzmann’s equation, it is possible to calculate the populations of atoms at these two levels (or at any other level). The energy of the upper level is 20.66 eV (or 3.31 × 10-18 J). Knowing that the photon is at 632.8 nm, we calculate the lower level to be at 2.99 × 10-18 J, and the rest is trivial. As expected, the population of atoms at the 20.66-eV level for neon in thermal equilibrium is extremely low, with N/N0 < 10-100 for room temperature neon. Even if there were a few atoms at these two levels involved in the lasing transition, there would still be many more at the lower level than at the upper level.
In an operating laser the upper level is selectively pumped with energy so that (1) there are many more atoms at the upper level than when at equilibrium, and (2) there is a higher population of atoms at the upper level than at the lower level. Because excited helium atoms (Figure 1.2) transfer energy to the 20.66-eV neon level and not the 18.69-eV level, the lower level will not fill from collisions the way the upper level does (the level for helium is not at all close to the 18.69-eV level). This situation produces the inversion we are seeking. It is no accident that helium is used in a He Ne laser: Helium has an excited energy level very close to the level of neon used as the upper level for the lasing transition. Direct electrical discharge in pure neon will not excite the upper level enough to cause an inversion (the lower levels would fill as well in that case). It must also be noted that the neon never ionizes in the He Ne laser described (i.e., loses an electron). Neon atoms in the He Ne laser remain neutral; they just acquire more energy, and a valence electrode is promoted to a higher orbital. Examination of the electron configuration for the upper energy level (2p5 5s1) reveals that all six valence electrons are still intact. In some lasers, the energy levels involved in lasing occur in the ion of the atomic species, but in this particular case it remains a neutral (but excited) atom. Ionized neon will lase under the right conditions, but the neon-ion laser is completely different in mechanism and spectral characteristics from the common He Ne laser.



|
|
|
|
5 علامات تحذيرية قد تدل على "مشكل خطير" في الكبد
|
|
|
|
|
|
|
لحماية التراث الوطني.. العتبة العباسية تعلن عن ترميم أكثر من 200 وثيقة خلال عام 2024
|
|
|