


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 27-8-2018
Date: 21-1-2022
Date: 18-5-2017
|
1,2-Group Shifts
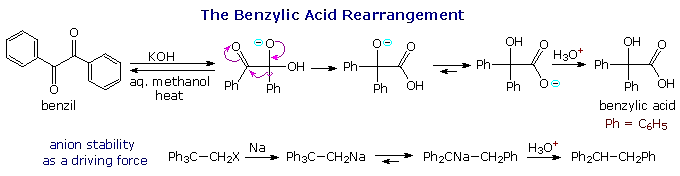
Two examples of 1,2-phenyl shifts are shown in the following diagram. In each case the driving force for the rearrangement is the conversion of a less stable anion into a more stable one. The reversible addition of hydroxide ion to one of the benzil carbonyl groups produces an intermediate which undergoes a pinacol-like rearrangement. In contrast to the carbocation "pull" that initiates the pinacol rearrangement, this benzylic acid rearrangement complements a weak electrophilic pull by the adjacent carbonyl carbon with the "push" of the alkoxide anion. A rapid proton transfer then forms the relatively stable carboxylate anion. In the second example, a very reactive 1º-carbanion (pKa ≈ 45) is converted to a diphenyl resonance stabilized carbanion (pKa ≈ 34).
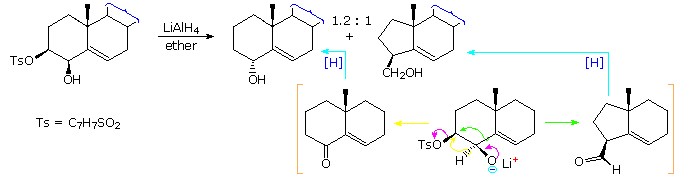
Similar 1,2-shifts of hydrogen or alkyl groups may also be favored by loss of a stable anion, as illustrated by the following example. Once again, an alkoxide anion provides a "push", and loss of the stable tosylate leaving group terminates the rearrangement. The LiAlH4 reagent not only generates the oxy-anion, but also reduces the resulting carbonyl products to alcohols. This rearrangement contrasts with the Wagner-Meerwein rearrangement in which a stable anion leaving group initiates the process by generating a carbocation species.
Brook Rearrangement
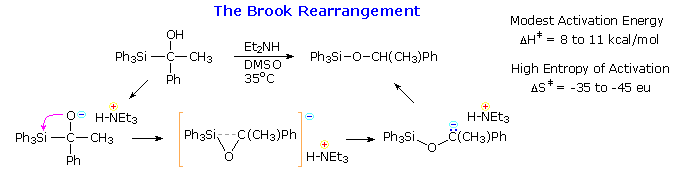
The rearrangement of silicon groups from carbon to oxygen is called the Brook rearrangement. An important driving force for this shift is the increased bond strength of the Si–O bond (110 Kcal/mol) compared with the Si–C bond (76 Kcal/mol). The example given in the following equation is catalyzed by base, and a cyclic transition state is indicated by the high entropy of activation. Retention of configuration at the silicon atom during the rearrangement was demonstrated by the reaction sequence shown by clicking on the diagram. Since the benzylation of the silyl chloride is known to proceed with inversion, and the final hydride reduction with retention, the rearrangement itself must have occurred with retention. Note that the sign of optical rotation for different silyl derivatives does not necessarily correlate with their configuration.
By clicking on the above diagram a third time, thermal 1,2 and 1,3- silicon rearrangements to oxygen in silyl esters and ketones will be displayed. When alkoxide base is added to silyl ketones, hypervalent silicon intermediates may be formed prior to rearrangement, as shown by one last click on the diagram. Mechanisms for the formation of various products are given by the curved arrows.
Reference
http://www2.chemistry.msu.edu/faculty/reusch/VirtTxtJml/rearrang.htm#top2



|
|
|
|
التوتر والسرطان.. علماء يحذرون من "صلة خطيرة"
|
|
|
|
|
|
|
مرآة السيارة: مدى دقة عكسها للصورة الصحيحة
|
|
|
|
|
|
|
نحو شراكة وطنية متكاملة.. الأمين العام للعتبة الحسينية يبحث مع وكيل وزارة الخارجية آفاق التعاون المؤسسي
|
|
|