


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 30-11-2015
Date: 21-4-2021
Date: 17-5-2016
|
Bicoid
Mutational analysis in the fruitfly Drosophila melanogaster has demonstrated that many aspects of anteroposterior patterning are already programmed in the oocyte by maternal gene activity (1, 2). During embryonic development, cells within an embryonic field receive positional information and are instructed by the concentration gradient of morphogens (3). Historically, the graded distribution of the Bicoid protein (Bcd) has provided the first evidence for the existence of such a morphogenetic gradient. Maternally derived bcd messenger RNA is localized to the anterior pole of the oocyte, where it serves as the source for the Bcd concentration gradient. Then, Bcd acts as a concentration-dependent activator of transcription and as a translational repressor. However, Bcd is only part of a wider regulatory network of maternal determinants, and its presence might actually be restricted to dipteran flies.
0.1.Anterior Localization of the Maternal Determinant Bicoid
Evidence for an anterior organizing center has been obtained by experimental embryology, for example, the removal and transplantation of polar cytoplasm (4). The nature of the patterning agents has been identified as RNA-containing particles (5). Genetic experiments have shown that the anterior morphogenetic function requires the activity of the bicoid maternal gene )bcd). Mothers homozygous mutant for bcd are normal, but all of their progeny lack head, thorax, and some abdominal structures. Instead posterior terminal structures are duplicated at the anterior ) Fig. 1; (6)). Cloning of the bcd gene revealed that bcd is expressed during oogenesis and that its mRNA is localized to the anterior tip of the oocyte and early embryo (Fig. 2) (7). This localization depends on the presence of the bcd 3′-UTR, which contains a specific structure recognized by factors that carry it to the anterior pole along the polarized microtubule array of the oocyte (8).

Figure 1. Phenotype of bcd embryos. Mothers homozygous mutant for bcd lay embryos that develop cuticles lacking all head structures (hs), thorax (T1, T2, T3), and two abdominal segments (A2, A4). Furthermore, the posterior structures like the spiracles (sp) are duplicated at the anterior.
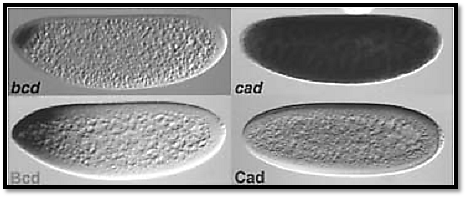
Figure 2. mRNA and protein expression of bcd and cad. The bcd mRNA is localized at the most anterior tip of the embryo. Its translation and diffusion of its protein product lead to the formation of the Bcd morphogenetic gradient. Maternal cad mRNA is distributed throughout the embryo, but its translation is blocked by the Bcd protein. This leads to the formation of a posterior to anterior gradient of the Cad protein.
0.2.The Morphogenetic Gradient of the Bicoid Transcription Factor
After egg deposition, the anteriorly localized bcd mRNA starts to be translated, producing a source of the Bcd protein. Then, Bcd diffuses passively in the syncytial environment of the blastoderm embryo and generates an anteroposterior concentration gradient whose high point is at the anterior pole of the embryo and whose low point is near the posterior pole (Fig. 2; (9, 10)). Decreasing or increasing the copy number of the bcd gene changes the slope of the Bcd gradient and, consequently, changes the fate map of the early embryo. This suggests that anterior positional values are specified by Bcd in a concentration-dependent manner (9, 10).
The Bcd protein contains a homeodomain (7) that does not belong to any specific class. It is characterized by a lysine residue at residue 50 (Lys50) of the homeodomain that determines its DNA-binding specificity (14). Bcd functions as a transcriptional activator in cell culture and in yeast (11-13). The morphogenetic gradient of Bcd differentially activates the first zygotically active segmentation genes, the gap genes. At high concentrations of Bcd, the head gap genes orthodenticle (otd), empty spiracles (ems), and buttonhead (btd) are activated (15-18), whereas lower levels of Bcd are required to activate hunchback (hb), which is expressed in a broad anterior domain (Fig. 3; (13)). A detailed study of the proximal hb promoter suggested that the affinity of Bcd binding sites in the promoters of the different target genes determines at which threshold level, and therefore at which position along the anterior-posterior axis, these genes are activated (19, 20). The ability of Bcd to activate target genes differentially provided the first molecular explanation of how a morphogen might act.

Figure 3. Expression pattern of bcd target genes. bcd is a maternal gene that controls the expression of the zygotic gap target genes. The morphogenetic gradient of Bcd protein differentially activates head (otd, ems, and btd) or thoracic (hb) gap genes.
Although Bcd is a DNA-binding protein, it also controls translation of the caudal gene (cad). cad is a gene required for posterior development, and its function is evolutionarily conserved in diverse organisms, including vertebrates (21). Maternal cad mRNA is ubiquitously distributed (22-24). Its translation is blocked at the anterior of the embryo by bcd, leading to a posterior-to-anterior gradient of the Cad protein (Fig. 3). cad is expressed ectopically in bcd mutant embryos throughout the embryo. Bcd binds to a sequence in the 3′-untranslated region of the cad mRNA (25-27). Like DNA binding, Bcd's RNA binding is conferred by the homeodomain and depends on the presence of Lys50 in the homeodomain. The Bcd homeodomain is the only one that binds RNA (25, 27).
0.3. Bicoid is Integrated into a Network of Maternal Determinants
Bcd is not the only maternal morphogen involved in anteroposterior patterning. A morphogenetic gradient of the Hunchback protein (Hb) creates the correct polarity in the embryo in the absence of bcd. The anteroposterior Hb gradient is established by translational repression of ubiquitous maternal mRNA by the nanos posterior determinant (28-30). Bcd and Hb are redundant for abdominal segmentation because high levels of maternal Hb rescue the abdominal phenotype of bcd mutant embryos (28, 29) and the activation of the central gap gene Krüppel. At the anterior of the embryo, Bcd and Hb synergize to activate the anterior gap genes (31). Because each morphogen has instructive functions on its own, both must be removed from the embryo to disrupt the polarity of the embryo completely. In more posterior regions, Bcd is also redundant with cad for activating the gap gene knirps (32). This shows that bcd function is partially redundant with other maternal functions. It should be noted that maternal hb and cad functions are not essential for viability in the presence of bcd. It is likely that they represent remains of ancestral systems that patterned the embryo before the appearance of bcd.
At the anterior pole of the embryo, Bcd function is influenced by the activity of the terminal system, which involves locally activating the Torso (Tor) receptor tyrosine kinase (33). Tor activity leads to two opposite effects on Bcd function. Tor activity increases the activator function of Bcd by lowering the threshold level of Bcd where target genes are activated (18, 34, 35). At the anterior pole, however, Bcd function is blocked in a Tor-dependent manner, and most bcd target genes retract from the anterior pole where Bcd concentration is the highest (36). The effect of tor activity on Bcd function might be mediated by tor-dependent phosphorylation of Bcd (36), or it may be indirect and may involve other factors (34).
0.4. Evolutionary Considerations on Bicoid
There are several reasons to believe that the critical functions of bcd in patterning the anteroposterior axis of the Drosophila embryo (Fig. 4) may not be conserved throughout evolution. bcd is unlikely to represent an ancestral morphogen. No bcd homologous genes have been identified outside higher dipterans (38), despite its homeobox and its specific location in the Hox cluster (7). Moreover, bcd shows an unusually high rate of divergence for a homeobox gene (39), and its function is not even conserved within higher dipterans (38, 40). Therefore, it is reasonable to assume that bcd is the result of recent gene duplication in the Hox cluster of dipterans and that it evolves relatively freely. Therefore, although the role of Bcd in Drosophila represents a striking example of a powerful morphogenetic transcriptional factor, it is likely that this function is not conserved in other insects and may exist only to allow the Drosophila embryo to develop quickly.
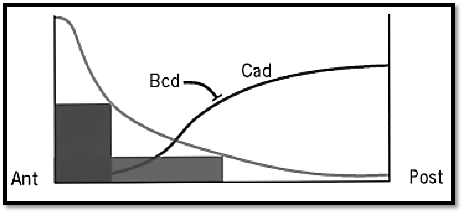
Figure 4. Morphogenetic functions of the Bcd gradient. The Bcd gradient differentially activates zygotic target genes (dark boxes) in head and thorax anlagen. The Bcd gradient also leads to the formation of the Cad protein gradient by blocking translation of its mRNA.
References
1.C. Nüsslein-Volhard and E. Wieschaus (1980) Nature 287, 795–801.
2.C. Nüsslein-Volhard, H. G. Frohnhofer, and R. Lehmann (1987) Science 238, 1675–1681.
3.L. Wolpert (1969) J. Theor. Biol. 25, 1–47.
4.K. Sander (1969) Advances in Insect Physiology (J. E. Treherne, M. J. Berridge, and V. B. Wigglesworth, eds.), Academic Press. Vol. 12, pp. 125–235.
5.K. Kalthoff (1979) Determinants of Spatial Organization (S. Subtelney and I. R. Konisberg, eds.), Academic Press, N.Y., pp. 97–126.
6.H. G. Fröhnhofer and C. Nüsslein-Volhard (1986) Nature 324, 120–125.
7.T. Berleth, M. Burri, G. Thoma, D. Bopp, S. Richstein, G. Frigerio, M. Noll, and C. Nüsslein-Volhard (1988) EMBO J. 7, 1749–1756.
8.D. Ferrandon, L. Elphick, C. Nüsslein-Volhard, and D. St Johnston (1994) Cell 79, 1221–1232.
9.W. Driever and C. Nüsslein-Volhard (1988) Cell 54, 95–104.
10.W. Driever and C. Nüsslein-Volhard (1988) Cell 54, 83–93.
11.S. D. Hanes and R. Brent (1989) Cell 57, 1275–1283.
12.W. Driever, J. Ma, C. Nüsslein-Volhard, and M. Ptashne (1989) Nature 342, 149–154.
13.G. Struhl, K. Struhl, and P. M. Macdonald (1989) Cell 57, 1259–1273.
14.J. Treisman and C. Desplan (1989) Nature 341, 335–337.
15.D. Dalton, R. Chadwick, and W. McGinnis (1989) Genes and Devel. 3, 1940–1956.
16.R. Finkelstein and N. Perrimon (1990) Nature 346, 485–488.
17. U. Walldorf and W. J. Gehring (1992) EMBO J. 11, 2247–2259.
18.E. A. Wimmer, M. Simpson-Brose, S. M. Cohen, C. Desplan, and H. Jäckle (1995(Mechanisms of Development 53, 235–245.
19.W. Driever, G. Thoma, and C. Nüsslein-Volhard (1989) Nature 340, 363–367.
20.G. Struhl (1989) Nature 338, 741–744.
21.V. Subramanian, B. I. Meyer, and P. Gruss (1995) Cell 83, 641–653.
22.M. Mlodzik and W. J. Gehring (1987) Cell 48, 465–478.
23.M. Mlodzik, A. Fjose, and W. J. Gehring (1985) EMBO J. 4, 2961–2969.
24.P. M. MacDonald and G. Struhl (1986) Nature 324, 537–545.
25.R. Rivera-Pomar, D. Niessing, U. Schmidt-Ott, W. J. Gehring, and H. Jäckle (1996) Nature 379, 746–749.
26.J. Dubnau and G. Struhl (1996) Nature 379, 694–699.
27.S. K. Chan and G. Struhl (1997) Nature 388, 634.
28.G. Struhl, P. Johnston, and P. A. Lawrence (1992) Cell 69, 237–249.
29.M. Hülskamp, C. Pfeifle, and D. Tautz (1990) Nature 346, 577–580.
30.C. Schülz and D. Tautz (1995) Development 121, 1023–1028.
31.M. Simpson-Brose, J. Treisman, and C. Desplan (1994) Cell 78, 855–865.
32.R. Rivera-Pomar, X. Lu, N. Perrimon, H. Taubert, and H. Jackle (1995) Nature 376, 253–256.
33.N. Perrimon and C. Desplan (1994) Trends in Biochemical Sciences 19, 509–513.
34.Q. Gao, Y. Wang, and R. Finkelstein (1996) Mechanisms of Development 56, 3–15.
35.U. Grossniklaus, K. M. Cadigan, and W. J. Gehring (1994) Development 120, 3155–3171.
36.E. Ronchi, J. Treisman, N. Dostatni, G. Struhl, and C. Desplan (1993) Cell 74, 347–355.
37.Y. Bellaïche, R. Bandyopadhyay, C. Desplan, and N. Dostatni (1996) Development 122, 3499-3508.
38.R. Schöder and K. Sander (1993) Roux''s Arch. Dev. Biol. 203, 34–43.
39.R. Sommer and D. Tautz (1991) Development 113, 419–430.
40.F. Bonneton, P. J. Shaw, C. Fazakerley, M. Shi, and G. A. Dover (1997) Mechanisms of Development 66, 143–156.



|
|
|
|
للعاملين في الليل.. حيلة صحية تجنبكم خطر هذا النوع من العمل
|
|
|
|
|
|
|
"ناسا" تحتفي برائد الفضاء السوفياتي يوري غاغارين
|
|
|
|
|
|
|
نحو شراكة وطنية متكاملة.. الأمين العام للعتبة الحسينية يبحث مع وكيل وزارة الخارجية آفاق التعاون المؤسسي
|
|
|