


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 17-11-2015
Date: 17-11-2015
Date: 17-11-2015
|
Primary Mycoses
Primary systemic mycoses include histoplasmosis (Histoplasma capsula- tum), North American blastomycosis (Blastomyces dermatitidis), coccidioidomycosis (Coccidioides immitis), and South American blastomycosis (Paracoccidioides brasiliensis). The natural habitat of these pathogens is the soil. Their spores are inhaled with dust, get into the lungs, and cause a primary pulmonary mycosis. Starting from foci in the lungs, the organisms can then be transported, hematogenously or lymphogenously, to other organs including the skin, where they cause granulomatous, purulent infection foci. Laboratory diagnostics aim at direct detection of the pathogens under the microscope and in cultures as well as identification of antibodies. The therapeutics used to treat these infections is amphotericin B and azoles. All of the primary systemic mycoses are endemic to certain geographic areas, in some cases quite limited in extent. Central Europe is not affected by these diseases. They are not communicable among humans.
Histoplasma capsulatum (Histoplasmosis)
Histoplasma capsulatum is the pathogen responsible for histoplasmosis, an intracellular mycosis of the reticuloendothelial system. The sexual stage or form of this fungus is called Emmonsiella capsulata.
Morphology and culture. H. capsulatum is a dimorphic fungus. As an infectious pathogen in human tissues it always forms yeast cells (Fig. 6.1). The small individual cells are often localized inside macrophages and have a diameter of 2-3 µm.
Giemsa and gram staining do not “take” on the cell walls of H. capsulatum, for which reason the cells often appear to be surrounded by an empty areola, which was incorrectly taken to be a capsule, resulting in the designation H. capsulatum. This species can be grown on the nutrient mediums normally used for fungal cultures. H. capsulatum grows as a mycelium in two to three weeks on Sabouraud agar at a temperature of 20-30 °C.
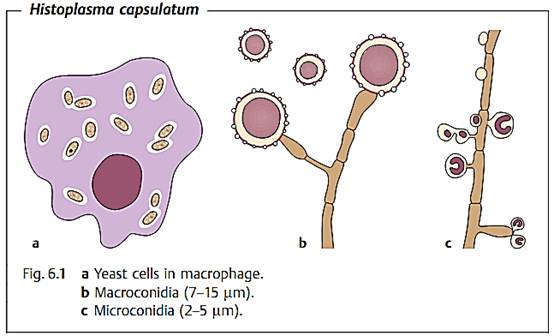
Pathogenesis and clinical picture. The natural habitat of H. capsulatum is the soil. Spores (conidia) are inhaled into the respiratory tract, are taken up by alveolar macrophages, and become yeast cells that reproduce by budding. Small granulomatous inflammatory foci develop. The pathogens can disseminate hematogenously from these primary infection foci. The reticuloendothelial system (RES) is hit particularly hard. Lymphadenopathies develop and the spleen and liver are affected. Over 90% of infections remain clinically silent. The clinical picture depends heavily on any predisposing host factors and the infective dose. A histoplasmosis can also run its course as a respiratory infection only. Disseminated histoplasmoses are also observed in AIDS patients.
Diagnosis. Suitable material for diagnostic analysis is provided by bronchial secretion, urine, or scrapings from infection foci. For microscopic examination, Giemsa or Wright staining is applied and yeast cells are looked for inside the macrophages and polymorphonuclear leukocytes. Cultures on blood or Sabouraud agar must be incubated for several weeks. Antibodies are detected using the complement fixation test and agar gel precipitation. The diagnostic value of positive or negative findings in a histoplasmin scratch test is doubtful.
Therapy. Treatment with amphotericin B is only indicated in severe infections, especially the disseminated form.
Epidemiology and prevention. Histoplasmosis is endemic to the midwestern USA, Central and South America, Indonesia, and Africa. With few exceptions, Western Europe is free of the disease. The pathogen is not communicable among humans. No special prophylactic measures are taken.
Coccidioides immitis (Coccidioidomycosis)
Morphology and culture. C. immitis is an atypical dimorphic fungus. In cultures, this fungus always grows in the mycelial form; in body tissues, however, it neither buds nor produces mycelia. What is found in vivo are spherical structures (spherules) with thick walls and a diameter of 15-60 µm, each filled with up to 100 spherical-to-oval endospores.
C. immitis is readily cultivated on the usual fungus nutrient mediums. After five days of incubation, a white, wooly (fuzzy) mycelial colony is observed. One of the morphological characteristics of the mycelium is the asexual arthrospores seen as separate entities among the hyphae.
Pathogenesis and clinical picture. The infection results from inhalation of dust containing arthrospores. Primary coccidioidomycosis is always localized in the lungs, whereby the level of manifestation varies from silent infections (60% of infected persons) to severe pneumonia. Five percent of those infected develop a chronic cavernous lung condition. In fewer than 1 %, hematogenous dissemination produces granulomatous lesions in skin, bones, joints, and meninges.
Diagnosis. The available tools are pathogen detection in sputum, pus, cerebrospinal fluid or biopsies, and antibody identification. The spherules can be seen under the microscope in fresh material. The fungus can be readily cultured on Sabouraud agar at 25 °C. The resulting arthrospores are highly infectious and must be handled very carefully. Antibodies can be detected using the complement fixation test, gel precipitation or latex agglutination. A coccidioidin skin test measuring any cellular allergy to components of the fungus is used as an initial orientation test if an infection is suspected.
Therapy. Amphotericin B can be used to treat the disseminated forms. An oral azole derivative will serve as an alternative, or for use, in clinically less severe forms.
Epidemiology and prevention. Coccidioidomycosis is endemic to desert areas of California, Arizona, Texas, New Mexico, and Utah and is only rarely observed elsewhere. The source of infection is the fungus-rich soil. Animals can also be infected. This disease is not transmitted among humans or from animals to humans.
Blastomyces dermatitidis (North American Blastomycosis)
Blastomyces dermatitidis is a dimorphic fungus that causes a chronic granulomatous infection. The pathogens occur naturally in the soil and are transmitted to humans by inhalation.
The primary blastomycosis infection is pulmonary. Secondary hematogenous spread can lead to involvement of other organs including the skin. Laboratory diagnostic methods include microscopy and culturing to identify the fungus in sputum, skin lesion pus, or biopsy material. Antibody detection using the complement fixation test or agar gel precipitation is of limited diagnostic value. Amphotericin B is the therapeutic agent of choice. Untreated blastomycoses almost always have a lethal outcome.
Blastomycosis occurs mainly in the Mississippi Valley as well as in the eastern and northern USA. Infections are also relatively frequent in animals, especially dogs. Susceptible persons cannot, however, be infected by infected animals or humans. There are no prophylactic measures.
Paracoccidioides brasiliensis (South American Blastomycosis)
Paracoccidioides brasiliensis (syn. Blastomyces brasiliensis) is a dimorphic fungus that, in living tissues, produces thick-walled yeast cells of 10-30 µm in diameter, most of which have several buds. When cultivated (25 °C), the fungus grows in the mycelial form.
The natural habitat of P. brasiliensis is probably the soil. Human infections are caused by inhalation of spore-laden dust. Primary purulent and/or granulomatous infection foci are found in the lung. Starting from these foci, the fungus can disseminate hematogenously or lymphogenously into the skin, mucosa, or lymphoid organs. A disseminated paracoccidioidomycosis progresses gradually and ends lethally unless treated. The therapeutic agents of choice are azole derivatives (e.g., itraconazole), amphotericin B, and sulfonamides. Therapy can prevent the disease from progressing, although no cases are known in which the disease is eliminated over the longer term. Laboratory diagnostics are based on detection of the pathogen under the microscope and in cultures as well as on antibody detection with the complement fixation test or gel precipitation.
Paracoccidioidomycosis is observed mainly among farmers in rural parts of South America.
References
Kayser, F. H. (2005). Medical Microbiology. Thieme Stuttgart. New York.



|
|
|
|
دخلت غرفة فنسيت ماذا تريد من داخلها.. خبير يفسر الحالة
|
|
|
|
|
|
|
ثورة طبية.. ابتكار أصغر جهاز لتنظيم ضربات القلب في العالم
|
|
|
|
|
|
|
قسم شؤون المعارف ووفد من جامعة البصرة يبحثان سبل تعزيز التعاون المشترك
|
|
|