
General Considerations of Infrared Spectroscopy
 المؤلف:
John D. Roberts and Marjorie C. Caserio
المؤلف:
John D. Roberts and Marjorie C. Caserio
 المصدر:
Basic Principles of Organic Chemistry : LibreTexts project
المصدر:
Basic Principles of Organic Chemistry : LibreTexts project
 الجزء والصفحة:
........
الجزء والصفحة:
........
 10-1-2022
10-1-2022
 3304
3304
General Considerations of Infrared Spectroscopy
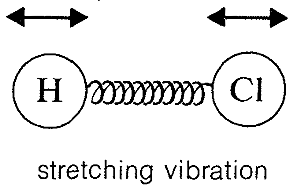
Absorption of infrared radiation causes transitions between vibrational energy states of a molecule. A simple diatomic molecule, such as H−Cl, has only one vibrational mode available to it, a stretching vibration somewhat like balls on the ends of a spring:
Molecules with three or more atoms can vibrate by stretching and also by bending of the chemical bonds, as indicated below for carbon dioxide:
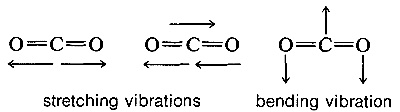
The absorption frequencies in the infrared spectra of molecules correspond to changes in the stretching or bending vibrations or both. In general, a polyatomic molecule with nn atoms will have 3n−6 modes of vibration of which n−1 are stretching vibrations and 2n−5 are bending vibrations. There are circumstances, however, where fewer vibrational modes are possible. If the molecule is linear, like CO2, then there are 3n−5 possible vibrations, and some of these vibrations may be equivalent (degenerate vibrations in the language of spectroscopists). For example, CO2 should have 3n−5 or vibrational modes, two of which are stretching and two of which are bending modes. However, the two bending modes are equivalent because the direction in which the molecule bends is immaterial; in-plane or out-of-plane bending are the same:
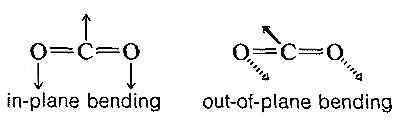
Diatomic molecules such as HCl have one vibrational mode, but it is important to note that symmetrical diatomic molecules, such as O2, N2, Cl2, F2, and H2, do not absorb in the infrared region of the spectrum. This is because absorption cannot occur if the vibration is electrically symmetrical. Fortunately, then, the infrared spectra can be recorded in air because the main components of air, N2 and O2, do not interfere.
In practice, infrared spectra can be obtained with gaseous, liquid, or solid samples. The sample containers (cells) and the optical parts of the instrument are made of rock salt (NaCl) or similar material that transmits infrared radiation (glass is opaque).
Typical infrared spectra are shown in Figure 9-9 for 2-propanone (acetone), CH3−CO−CH3, and 2-butanone (methyl ethyl ketone), CH3−CO−CH2−CH3. In accord with current practice, the position of absorption (horizontal scale) is recorded in units of wave numbers (ν¯, cm−1). The vertical scale measures the intensity of radiation transmitted through the sample. Zero transmission means complete absorption of radiation by the sample as at 1740cm−1 in Figure 9-9. The other absorption bands in Figure 9-9 that correspond to excitation of stretching or bending vibrations are not as intense as the absorption at 1740cm−1.
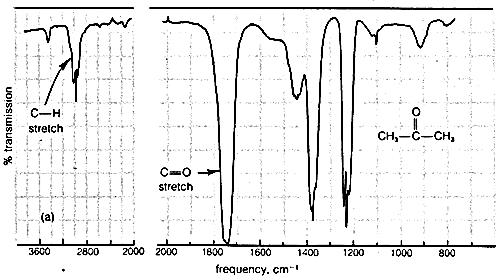

Figure 9-9: Infrared absorption spectra of (a) 2-propanone and (b) 2-butanone in the vapor phase.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


