

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Why Can‘t We See Molecules?
المؤلف:
John D. Roberts and Marjorie C. Caserio
المصدر:
Basic Principles of Organic Chemistry : LibreTexts project
الجزء والصفحة:
........
9-1-2022
2788
Why Can't We See Molecules?
The most straightforward way to determine the structures of molecules would be to "see" how the nuclei are arranged and how the electrons are distributed. This is not possible with visible light, because the wavelengths of visible light are very much longer than the usual molecular dimensions. A beam of electrons can have the requisite short wavelengths, but small organic molecules are destroyed rapidly by irradiation with electrons of the proper wavelengths. Nonetheless, electron microscopy is a valuable technique for the study of large molecules, such as DNA, which can be stained with heavy-metal atoms before viewing, or are themselves reasonably stable to an electron beam (Figures 9-4 and 9-5).
Figure 9-4: Electron micrograph (x40,000) of two linked (catenated) cyclic mitochondrial DNA molecules from a culture of human cells. The DNA was stained with uranyl acetate, then shadowed with platinum and palladium atoms in high vacuum to make the molecules easily visible in the electron microscope. (Photograph supplied by Dr. B. S. Hudson and the late Dr. J. Vinograd.)
Figure 9-5: Electron micrograph (x150,000) of a thin layer of copper hexadecachlorophthalocyanine molecules. The molecules are tilted about 26o26o from the horizontal plane. (Courtesy JEOL, Ltd.)
Virtually all parts of the spectrum of electromagnetic radiation, from x rays lo radio waves, have some practical application for the study of organic molecules. The use of x-ray diffraction for determination of the structures of molecules in crystals is of particular value, and in the past ten years this technique has become almost routine. Figure 9-6 shows the detailed arrangement of the carbons, hydrogens, and bromines in 1,8-bis(bromomethyl)naphthalene, 1, as determined by x-ray diffraction. The apparatus and techniques used are highly complex and are not available yet to very many organic laboratories.
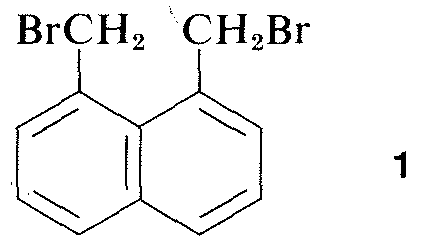
Other diffraction methods include electron diffraction, which may be used to’determine the structures of gases or of volatile liquid substances that cannot be obtained as crystals suitable for x-ray diffraction, and neutron diffraction, which has special application for crystals in which the exact location of hydrogens is desired. Hydrogen does not have sufficient scattering power for x rays to be located precisely by x-ray diffraction.
Figure 9-6: Bond lengths, angles, and arrangement of carbons and bromines in a crystal of 1,8-bis(bromomethyl)naphthalene, 1, as determined by x-ray diffraction. Notice that the preferred conformation in the crystal has the bromines on opposite sides of the naphthalene ring.
The diffraction methods can be used to determine complete structures of organic molecules, but they are not sufficiently routine to be utilized generally in practical organic laboratory work. For this reason, in the remainder of this chapter we will emphasize those forms of spectroscopy that are generally available for routine laboratory use. As will be seen, these methods are used by organic chemists in more or less empirical ways. In general, spectroscopic methods depend on some form of excitation of molecules by absorption of electromagnetic radiation and, as we have said, virtually all parts of the electromagnetic spectrum have utility in this regard. The commonly used span of the electromagnetic spectrum is shown in Figure 9-7 along with a comparison of the various units that are employed to express energy or wavelength.
The major kinds of spectroscopy used for structural analysis of organic compounds are listed in Table 9-1. The range of frequencies of the absorbed radiation are shown, as well as the effect produced by the radiation and specific kind of information that is utilized in structural analysis. After a brief account of the principles of spectroscopy, we will describe the methods that are of greatest utility to practical laboratory work.
You may have problems with the relationships among the variety of wavelength and frequency units commonly used in spectroscopy. The relationship between wavelength, frequency, and velocity should become clear to you by considering yourself standing on a pier watching ocean waves going by. Assuming the waves are uniformly spaced, there will be a uniform distance between the crests, which is λ, the wavelength. The wave crests will pass by at a certain number per minute, which is ν, the frequency. The velocity, c, at which the crests move by you is related to λ and ν by the relationship c=λνc.
This is not really very complicated and it applies equally well to water waves or electromagnetic radiation. What is almost needlessly complicated is the variety of units commonly used to express λ and ν for electromagnetic radiation. One problem is tradition, the other is the desire to avoid very large or very small numbers. Thus, as Figure 9-7 shows, we may be interested in electromagnetic wavelengths that differ by as much as a factor of 1016. Because the velocity of electromagnetic radiation in a vacuum is constant at 3×108 m sec−1 , the frequencies will differ by the same factor.
Figure 9-7: The span of the spectrum of electromagnetic radiation used in spectroscopic investigations of organic compounds along with comparison of some of the various units commonly employed for wavelength and energy of the radiation on log scales.
Units commonly used for wavelength are meters (m), centimeters (cm), nanometers (nm), and microns (μμ). In the past, angstroms (Å) and millimicrons (mμm) also were used rather widely.
1m=102cm=109nm=106μm (9.4.1)
10−2m=1cm=107nm=104μm (9.4.2)
10−6m=10−4cm=103nm=1μm (9.4.3)
10−9m=10−7cm=1nm=10−3μm=1mμm=10Å (9.4.4)
Frequency units are in cycles per second (cps) or hertz (Hz), which are equivalent (radians per second are used widely by physicists).
Table 9-1: Principal Spectroscopic Techniques Currently in Use for Analysis of Molecular Structure
Figure 9-7 generally to be large. As a result, it is common to use wave numbers instead of Hz or MHz (megahertz). The frequency in wave number is simply the frequency ν in Hz divided by c, the velocity of light in cmcm. Wave-number units are cm−1 and we can think of the wave number ν∼ as being the number of wave crests per centimeter.
1Hz=10−6 MHz ≅ 3.3×10−11cm−1 (9.4.5)
106 Hz=1MHz ≅ 3.3×10−5cm−1 (9.4.6)
3×1010 Hz= 3×104 MHz ≅1cm−1 (9.4.7)
A useful description of how molecular structures can be determined by "x-ray vision" is given in Chapter XI of Organic Molecules in Action by M. Goodman and F. Morehouse, Gordon and Breach, New York, 1973.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















