


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
أقرأ أيضاً
التاريخ: 29-9-2019
التاريخ: 21-12-2021
التاريخ: 1-10-2019
التاريخ: 3-4-2017
|
The reactivity of a given alkyl derivative, RX, in either SN1 or SN2 reactions, is influenced strongly by the leaving group, X. The choice of leaving group is therefore an important consideration in any synthesis involving SN reactions.
From the foregoing discussion of structural effects in the R group on SN reactivity, particularly in SN1 reactions, we might expect the stability of :X as an ion or neutral molecule to play a major role in determining how good or poor X is as a leaving group. The stability of :X is indeed important - the problem is that there are several factors that contribute to the stavility and hence the lability of the leaving group.
For the purpose of initially identifying good and poor leaving groups, consider development of a practical synthesis of diethyl ether. One route is by way of SN2 displacement using an ethyl compound, CH3CH2X, and ethoxide ion:
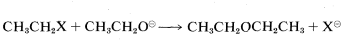
Many CH3CH2X compounds have X groups that are quite unsatisfactory in this reaction. They include compounds such as ethane, propane, ethanol, ethyl methyl ether, ethylamine, and ethyl ethanoate; the respective groups, H⊖, CH3⊖, HO⊖, CH3O⊖, NH2⊖, and CH3CO2⊖ all can be classified as very poor leaving groups. The more reactive ethyl derivatives (see Table 8-3) include the halides, particularly ethyl iodide, and sulfonic acid derivatives; the corresponding anions Cl⊖, Br⊖, I⊖, and RS(O2)O⊖ therefore are moderate to good leaving groups. Table 8-3 includes pertinent data for the rates of ether formation from various alkyl compounds and illustrates that the relative abilities of groups to leave are about the same in SN1 reactions as they are in SN2 reactions.
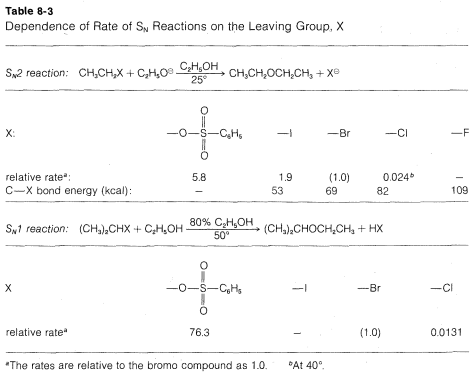
Why are groups such as I⊖ and RSO⊖ good leaving groups, whereas others such as H⊖, HO⊖ and NH2⊖ are poor? The simplest correlation is with the strength of HX as an acid. This is very reasonable because the ease of loss of X⊖, as from (CH3)C−X in an SN1 reaction, would be expected to be related, to some degree at least, to the ease of ionization of H−X to H⊕ and X⊖. Therefore the stronger HXHX is as an acid, the better X will be as a leaving group. Thus HFHF is a relatively weak acid and F⊖ is not a very good leaving group; H−I is a very strong acid and I⊖ is a good leaving group. The usual order of reactivity of alkyl halides, R−I > R−Br > R−Cl > R−F (when R is the same group throughout), is in accord with the acid strengths of the halogen acids. SImilarly, CF3CO2− is a much better leaving group than CH3CO2−, and we find that trifluoroethanoic acid, CF3CO2H is a several thousand times stronger acid than ethanoic acid, CH3CO2H. For the same reason, CF3SO3⊖ is a better leaving group than CH3XO3⊖.
This correlation can be extended easily to groups that leave as neutral X:. For example, ROH2⊕→R⊕+H2O occurs far more readily than ROH→R⊕+OH⊖ and we know that H3O⊕ is a stronger acid (or better proton donor) than H2O.
The relationship between X⊖ as a leaving group and HX as an acid is very useful because much information is available on acid strengths. However, it is not a very fundamental explanation unless we can explain why some acids are strong acids and others are weak acids. One factor is the strength of the H−X bond, but here we need to remember that the usual bond strengths are for dissociation to radicals or atoms, not ions, and for the gas, not for solutions. If we write the steps relating the bond-dissociation energy to the energy of ionic dissociation in solution, we see that for variations in X, in addition to the bond energy, the electron affinity of X⋅, the solvation energy of X⊖, and the solvation energy of HX, also will be contributing factors.
Figure 8-5 and Table 8-3).
Figure 8-5: Plot of C−X bond energies against H−X bond energies, using the data of Table 4-3



|
|
|
|
حقن الذهب في العين.. تقنية جديدة للحفاظ على البصر ؟!
|
|
|
|
|
|
|
علي بابا تطلق نماذج "Qwen" الجديدة في أحدث اختراق صيني لمجال الذكاء الاصطناعي مفتوح المصدر
|
|
|
|
|
|
|
مشاتل الكفيل تنتج أنواعًا مختلفة من النباتات المحلية والمستوردة وتواصل دعمها للمجتمع
|
|
|