

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
The Nature of the Solvent
المؤلف:
John D. Roberts and Marjorie C. Caserio
المصدر:
Basic Principles of Organic Chemistry : LibreTexts project
الجزء والصفحة:
........
5-1-2022
2330
The Nature of the Solvent
The rates of SN reactions are sensitive to the nature and composition of the solvent. This is easy to understand for SN1 reactions because the ionizing power of a solvent is crucial to the ease of formation of ions R⊕ and X⊖ from RX.
Actually, two factors are relevant in regard to the ionizing ability of solvents. First, a high dielectric constant increases ionizing power by making it easier to separate ions. This is because the force between charged particles varies inversely with the dielectric constant of the medium. Thus water, with a dielectric constant of 80, is 40 times more effective than a hydrocarbon with a dielectric constant of 2. Second, and usually more important, is the ability of the solvent to solvate the separated ions. Cations are solvated most effectively by compounds of elements in the first row of the periodic table that have unshared electron pairs. Examples are ammonia, water, alcohols, carboxylic acids, sulfur dioxide, and methylsulfinylmethane [dimethyl sulfoxide, (CH3)2SO]. Anoins are solvated most efficiently by solvents having hydrogen attached to a strongly elecronegative element Y so the H−Y bond is strongly polarized as Hδ⊕⋅⋅⋅Yδ⊖. Such solvents usually are called protic solvents. Protic solvents form hydrogen bonds to the leaving group, which assist ionization in much the same way that silver ion catalyzes ionization of alkyl halides. We can represent solvation by the following structural formulas, but it must be recognized that the number of solvent molecules involved in close interacitons can be as large as four or six, or as small as one:
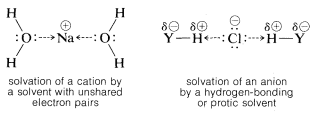
The most effective ionizing solvents are those that effectively solvate both anions and cations. Water strikes an excellent compromise with regard to the structural features that make up ionizing power, that is, dielectric constant and solvating ability. From this, we expect tert-butyl chloride to ionize much more readily in water than in ether, because ethers can solvate only cations effectively, whereas water can solvate both anions and cations. The fact is that SN1 ionizations usually are so difficult that SN1 reactions seldom occur in solvents that cannot effectively solvate both anions and cations, even if the dielectric constant of the solvent is high. Solvation by hydrogen bonding is especially helpful in assisting ionization. Solvents that cannot provide such hydrogen bonding [e.g., CH3OCH3, (CH3)3N, CH3NO2, CH3CN, (CH3)2SO] generally are poor for SN1 reactions. These solvents are called aprotic solvents. An important exception is liquid sulfur dioxide, SO2, which promotes SN1 ionization by having a high dielectric constant and being able to solvate both anions and cations.
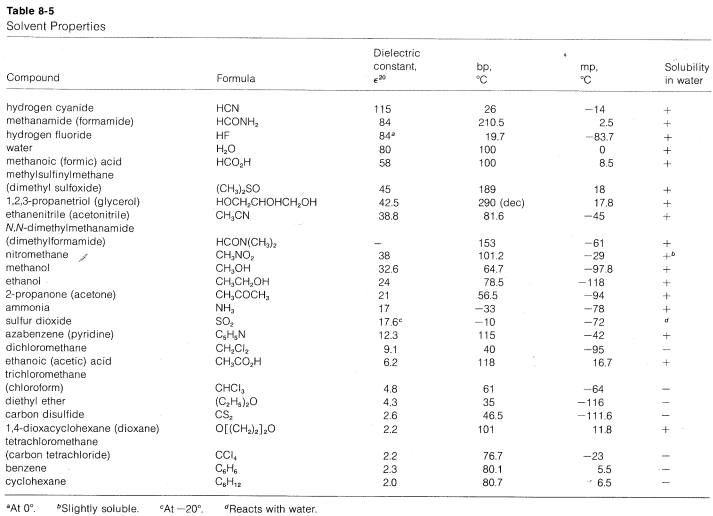
A list of protic and aprotic solvents, their dielectric constants, boiling points, and melting points is given in Table 8-5. This table will be useful in selecting solvents for nucleophilic substitution reactions.
With regard to SN2 reactions, the solvent can affect profoundly the reactivity of a given nucleophile. Thus anions such as Cl⊖ and CN⊖, which are weakly nucleophilic in hydroxylic solvents and in poor ionizing solvents such as 2-propanone (acetone), become very significantly nucleophilic in polar aprotic sovlents such as (CH3)2SO. The reason is that for salts such as NaCl and NaCN the aprotic solvent preferentially solvates the cation, leaving the anion relatively bare. This dissociation of the anion from the cation together with its poor solvation makes the anion abnormally reactive as a nucleophile.
The Greek letters α, β, γ are used here not as nomenclature, but to designated the positions along a carbon chain from a functional group, X: Cω⋯Cδ−Cγ−Cβ−Cα−X .
Specifically, electrostatic force =q1q2/r212ϵ in which q1 and q2 are the charges, r12 is the distance between the charges, and ϵ is the dielectric constant of the medium; ϵ=1 for a vacuum.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















