


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date:
Date: 12-7-2021
Date: 5-11-2015
|
Brain and Meninges
The brain is made up of a cerebrum (telencephalon and diencephalon), a cerebellum (metencephalon), and a brainstem (mesencephalon = midbrain; mesencephalon = pons; myelencephalon = medulla). Protective and physiologic membranous coverings, called meninges, surround structures of the brain.
A. Embryology
The central nervous system (CNS), that is, the brain and spinal cord, begins to form in week 3 of development, during which time the process of neurulation occurs (Fig. 1). Neurulation refers to the formation and closure of the neural tube in which BMP-4 (bone morphogenetic protein), noggin (an inductor protein), chordin (an inductor protein), FGF-8 (fibroblast growth factor), and N-CAM (neural cell adhesion molecule) play a role.
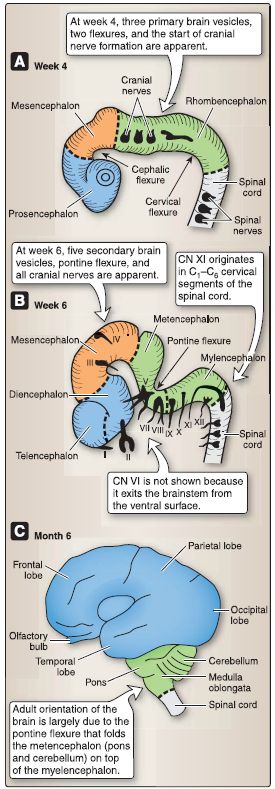
Figure 1: Embryology of the brain (A-C).
1. Brain development: Neurulation begins when the primitive node/ notochord induces the overlying ectoderm to form the neural plate, which is a thick plate of pseudostratified, columnar neuroepithelial cells called neuroectoderm. The neural plate is shaped with a broad cranial portion that gives rise to the brain and a narrow caudal portion that gives rise to the spinal cord.
Even before neurulation begins, the primordia of the three primary brain vesicles (i.e., prosencephalon, mesencephalon, and rhombencephalon) are apparent as broadenings of the neural plate. The three primary brain vesicles further develop into five secondary brain vesicles (i.e., telencephalon, diencephalon, mesencephalon, metencephalon, and myelencephalon). During week 5, the prosencephalon subdivides into the telencephalon and the diencephalon, and the rhombencephalon subdivides into the metencephalon and the myelencephalon. The mesencephalon does not subdivide. From these five secondary brain vesicles, the adult derivatives of the brain form.
a. Ventricles: Within each of the brain vesicles, the neural canal develops into the definitive ventricular system of the brain. The neural canal of the telencephalon becomes the paired lateral ventricles. The neural canal of the diencephalon becomes the third ventricle. The neural canal of the mesencephalon becomes the cerebral aqueduct. The neural canal of the metencephalon and myelencephalon (or the rhombencephalon) becomes the fourth ventricle.
b. Neural tube folding: During weeks 4-8, the neural tube folds sharply at three locations. The first fold that develops is a ventral folding called the cephalic flexure located at the mesencephalon.
The second fold that develops is a ventral folding called the cervical flexure located at the junction of the myelencephalon and the spinal cord. The third fold that develops is a dorsal folding called the pontine flexure located at the pons. Refer to Table 1 for additional details.
Table 1: Primary and Secondary Brain Vesicles
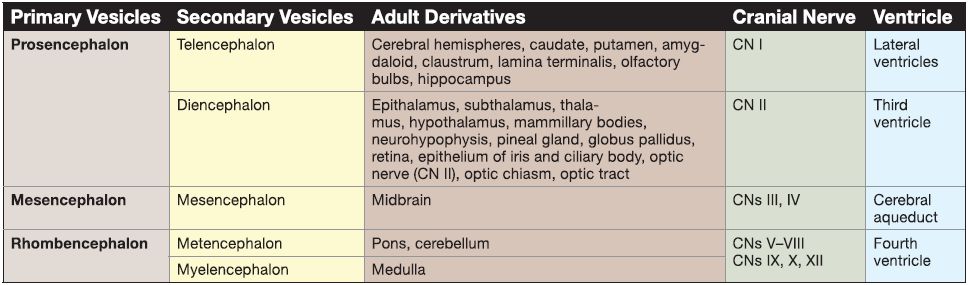
2. Neural tube histogenesis: The cells of the neural tube are derived from neuroectoderm that form neuroblasts and glioblasts. The neuroblasts form all the neurons in the CNS. The glioblasts form the supporting cells (or neuroglial cells) of the CNS. The two main types of supporting cells are the astrocytes and the oligodendrocytes.
The other important neuroglial cells in the CNS are the microglia. The microglia are actually macrophages within the CNS that arise from monocytes (i.e., mesoderm) that invade the developing nervous system in week 3 along with the developing blood vessels (Fig. 2).
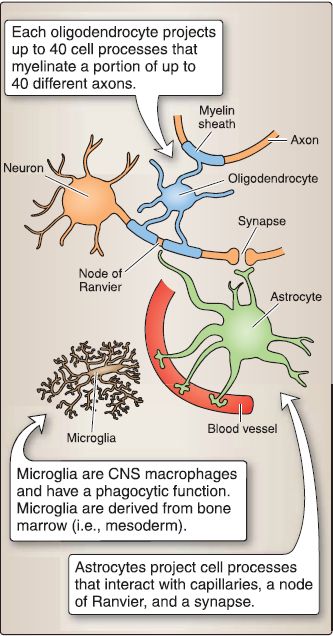
Figure 2: Histogenesis during brain development. CNS, central nervous system.
a. Astrocyte: Astrocytes project foot processes to capillaries that contribute to the blood-brain barrier and play a role in the metabolism of neurotransmitters (e.g., glutamate, y-aminobutyric acid, serotonin). Astrocytes buffer the [K+] of the CNS extracellular space and form the external and internal glial-limiting membrane in the CNS. In reaction to CNS injury, astrocytes undergo hypertrophy and hyperplasia and form glial scars in a damaged area of the CNS (i.e., astrogliosis). The astrocyte contains glial fibrillary acidic protein (GFAP) and glutamine synthetase, which are good markers for astrocytes. The two types of astrocytes are fibrous and protoplasmic.
[1] Fibrous astrocyte: Fibrous astrocytes are found predominately in the white matter, and about 80% of adult primary tumors arise from fibrous astrocytes.
[2] Protoplasmic astrocyte: Protoplasmic astrocytes are found predominately in the gray matter.
b. Oligodendrocyte: The oligodendrocyte may project up to 40 cell processes, each of which is involved in myelination. Each oligodendrocyte cell process produces a myelin sheath that surrounds and insulates a portion of only one axon. Because one oligodendrocyte may project up to 40 cells processes, it may myelinate a portion of up to 40 different axons. Because the junction between adjacent oligodendrocyte cell processes lacks myelin, this segment of the axon is exposed to the extracellular milieu due to gaps in the myelin sheath. This segment of the axon is called the node of Ranvier.
c. Microglia: Microglia are the smallest neuroglia and have a phagocytic function. They are derived from colony-forming unit-granulocyte monocyte cells found in the bone marrow (i.e., mesoderm). Microglia normally comprise 1 % of all the neuroglia in the CNS but proliferate in regions of injury or disease. These are then called reactive microglia.
3. Hypophysis development and histology: The hypophysis (or pituitary gland) forms from two distinct embryologic sources (Fig. 3). The first source is from ectoderm that lines the stomodeum (or the primitive mouth). The second source is from neuroectoderm associated with the diencephalon. The hypophysis comprises two embryologically distinct components, the adenohypophysis and the neurohypophysis (Fig. 4).
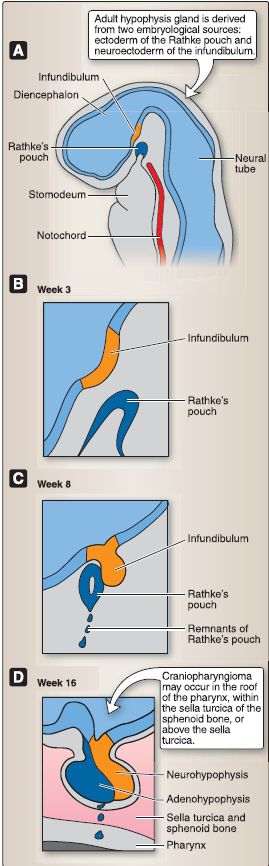
Figure 3: Hypophysis embryology (A-D).
 Figure 4: Hypophysis histology. A, Gross histology. B, Hypothalamo-hypophyseal portal system. C and D, Cell types. ACTH = adrenocorticotropic hormone, ADH = antidiuretic hormone, FSH = follicle-stimulating hormone, GH = growth hormone, IFs = inhibiting factors, LH = luteinizing hormone, OXY = oxytocin, PRL = prolactin, RFs = releasing factors, TSH = thyroid-stimulating hormone.
Figure 4: Hypophysis histology. A, Gross histology. B, Hypothalamo-hypophyseal portal system. C and D, Cell types. ACTH = adrenocorticotropic hormone, ADH = antidiuretic hormone, FSH = follicle-stimulating hormone, GH = growth hormone, IFs = inhibiting factors, LH = luteinizing hormone, OXY = oxytocin, PRL = prolactin, RFs = releasing factors, TSH = thyroid-stimulating hormone.
a. Adenohypophysis: At week 3, an upward evagination of ectoderm that lines the stomodeum called Rathke's pouch appears. Rathke's pouch grows dorsally toward the diencephalon and, by week 8, loses its connection with the stomodeum and comes into close contact with the infundibulum of the diencephalon. Rathke's pouch eventually forms the adenohypophysis, which includes three subdivisions: pars distalis, pars tuberalis, and pars intermedia.
[1] Pars distalis: The pars distalis contains the majority of endocrine cells, including somatotropes, mammotropes, corticotropes, gonadotropes, and thyrotropes. Table 2 provides a summary of hormones produced by these cells.
Table 2: Hormones of the Adenohypophysis and Neurohypophysis
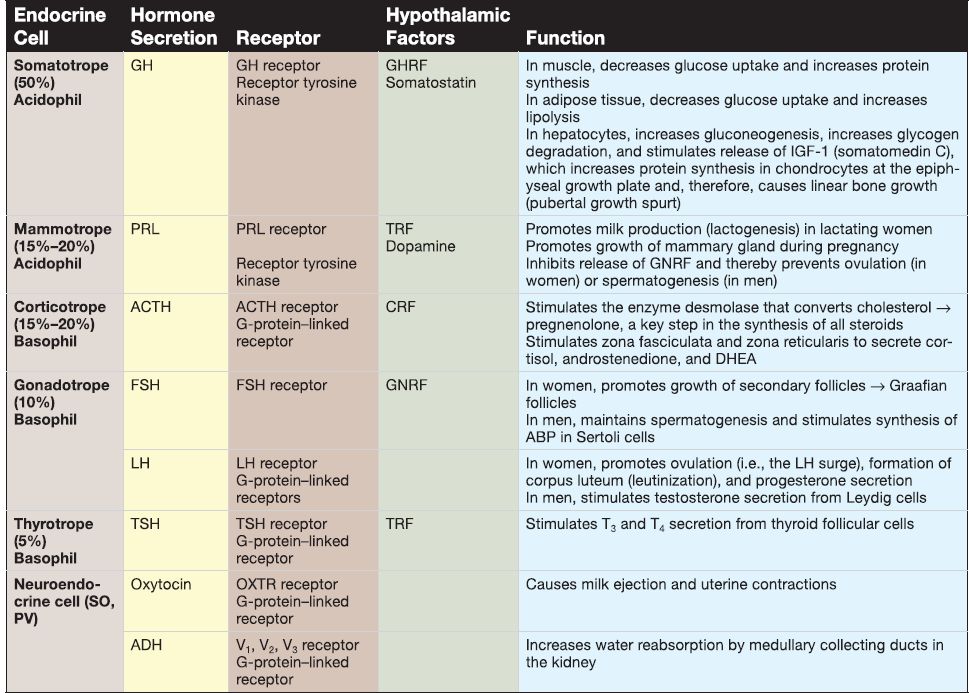
[2] Pars tuberalis: The pars tuberalis surrounds the median eminence and infundibular stem of the neurohypophysis. The par tuberalis contains some corticotropes and gonadotropes. In addition, the pars tuberalis contains the portal venules of the hypothalamo-hypophyseal portal system.
[3]. Pars intermedia: The pars intermedia (rudimentary in humans) contains numerous colloid-filled cysts called Rathke's cysts. b. Neurohypophysis: At week 3, a downward evagination of neuroectoderm associated with the diencephalon called the infundibulum appears. The infundibulum grows ventrally toward Rathke's pouch and, by week 8, maintains its connection to the diencephalon (specifically the hypothalamus) and comes into close contact with Rathke's pouch. The infundibulum eventually forms the neurohypophysis. The neurohypophysis consists of two subdivisions, the pars nervosa and the infundibulum.
[1] Pars nervosa: The pars nervosa contains the unmyelinated axons and axon terminals that belong to neuroendocrine cells whose cell bodies are located in the supraoptic nucleus and the paraventricular nucleus of the hypothalamus. The pars nervosa also contains glial-like cells called pituicytes. (a) Hormones: The cell bodies of the neuroendocrine cells located in the hypothalamic supraoptic and paraventricular nuclei synthesize oxytocin and antidiuretic hormone (ADH; see Table 2). Their unmyelinated axons project to the pars nervosa and carry large aggregations of neurosecretory vesicles called Herring bodies. The Herring bodies contain oxytocin or ADH along with the carrier protein neurophysin. The axon terminals of the neuroendocrine cells secrete oxytocin and ADH into a capillary plexus formed by the inferior hypophyseal artery.
(b) Pituicyte: Pituicytes are glial-like cells that resemble astrocytes. The pituicyte contains intermediate filaments assembled from GFAP. It has cell processes that project to fenestrated capillaries and the perivascular space.
[2] lnfundibulum: The infundibulum is a stalk that connects the pars nervosa to the hypothalamus.
B. Adult brain
The brain is made up of a cerebrum (telencephalon and diencephalon), brainstem (mesencephalon = midbrain; mesencephalon = pons; myelencephalon = medulla), and a cerebellum (metencephalon), as shown in Figure 5 .
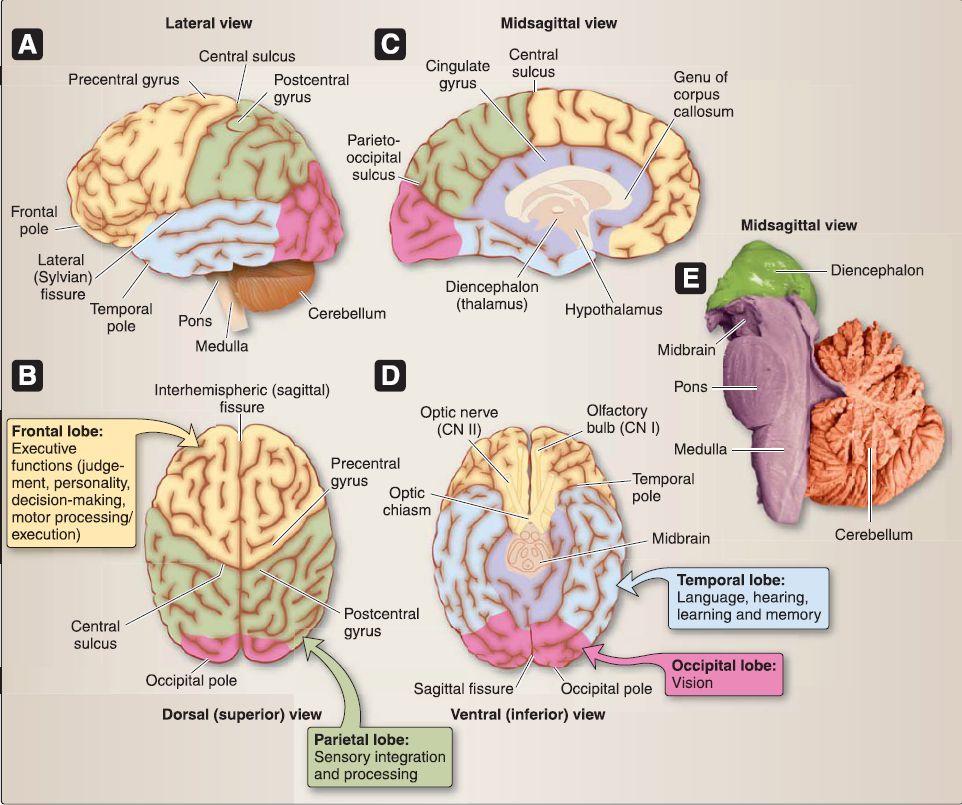 Figure 5 : Organization of the brain. A-D, Frontal lobe, parietal lobe, occipital lobe, temporal lobe, limbic lobe/system. E, Brainstem and cerebellum.
Figure 5 : Organization of the brain. A-D, Frontal lobe, parietal lobe, occipital lobe, temporal lobe, limbic lobe/system. E, Brainstem and cerebellum.
1. Cerebrum: The cerebrum is divided into right and left cerebral hemispheres, which are separated in the midline along a longitudinal fissure. The cerebral surface (cortex) is characterized by multiple gyri (folds) and sulci (grooves). This gyri/sulci arrangement allows for greater cortical surface area. A general organization of sulci and gyri is maintained within each hemisphere, although no two brains are identical.
a. Lobes: Each cerebral hemisphere is further divided into four lobes. Both right and left cerebral hemispheres have a frontal, parietal, temporal, and occipital lobe. Laterally, the temporal lobe is separated from the frontal and parietal lobes by the lateral fissure. The ventral surface of the cerebrum sits within the cranial base, so that the frontal lobes are located in the anterior cranial fossa and the temporal lobes in the middle cranial fossa.
b. Diencephalic structures: A midsagittal view of a cerebral hemisphere reveals diencephalic structures, including the thalamus, hypothalamus, pineal gland, and epithalamus.
2. Brainstem: The brainstem begins at the junction of the middle and posterior cranial fossae and extends to the foramen magnum where it continues inferiorly as the spinal cord. The midbrain is the most rostral portion of the brainstem, followed by the pons and medulla caudally. Multiple CNs arise from regions of the brainstem and is discussed in detail later in this chapter.
3. Cerebellum: The cerebellum occupies the remaining space in the posterior cranial fossa. Latin for "little brain," the cerebellum also possesses right and left hemispheres, which are united in the midline by the vermis.
B. Ventricular system
The ventricular system of the brain is made up of CSF-filled paired lateral ventricles and singular midline third and fourth ventricles (Fig. 6). The tuft-like choroid plexus found throughout the ventricular system is responsible for CSF production. Each ventricle is associated with a specific region of the brain: right and left lateral ventricles, telencephalon; third ventricle, diencephalon; and fourth ventricle, mesencephalon (pons/cerebellum) and myelencephalon (medulla).
 Figure 6: Ventricular system. A, Schematic views of ventricles {blue). B, Brain with ventricles in situ and cisterns. Note direction of cerebrospinal fluid (CSF) flow (red arrows) and relationship between arachnoid granulations and superior sagittal sinus.
Figure 6: Ventricular system. A, Schematic views of ventricles {blue). B, Brain with ventricles in situ and cisterns. Note direction of cerebrospinal fluid (CSF) flow (red arrows) and relationship between arachnoid granulations and superior sagittal sinus.
1. Cerebrospinal fluid flow: Flow through the ventricular system occurs normally as long at the ventricles can communicate freely. Flow occurs from the lateral ventricles, through the interventricular foramen (of Monro) into the third ventricle. A narrow conduit through the midbrain, called the cerebral aqueduct, connects the third and fourth ventricles. From the fourth ventricle, CSF either continues inferiorly into the central canal of the spinal cord or can flow out into the subarachnoid space through a median aperture (of Magendie) or through paired lateral apertures (of Lusaka).
2. Subarachnoid cisterns: From the ventral surface of the brain, CSF may pool in a series of enlarged subarachnoid spaces called cisterns, primarily localized adjacent to the cerebellum, brainstem, and ventral structures. CSF flows superiorly within the subarachnoid space, bathing the sulci and gyri and functioning to nourish and cushion the brain within the cranial vault. Circulating CSF within the cranium is absorbed into the dural venous sinus system by way of arachnoid granulations.
C. Cranial meninges
Like spinal meninges, cranial meninges collectively surround, support, and protect the brain within the cranium (Fig. 6). Dural layers serve both to protect the brain in the cranium and to form partitions and venous sinuses (Fig.7 ). From superficial to deep, these layers are described below.
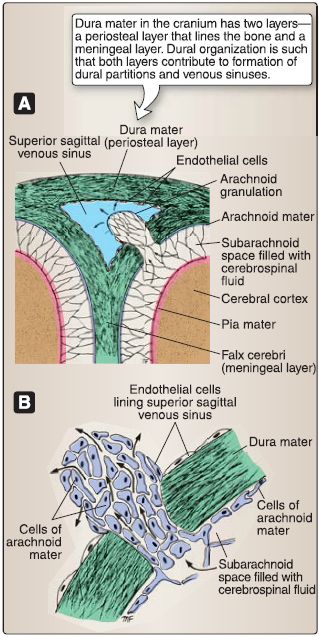
Figure 6: Meninges and arachnoid granulations. A, Relationship with superior sagittal sinus. B, Cellular organization.
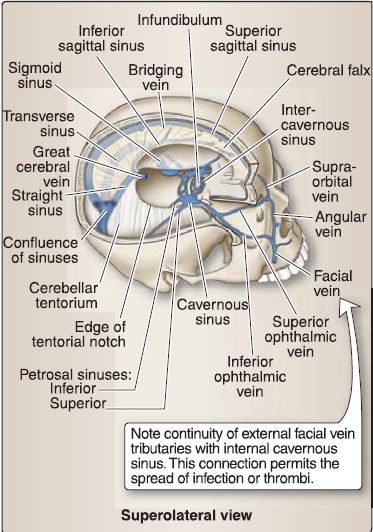
Figure 7: Dural reflections/sinuses: right calvarium and brain removed to view dural partitions {white) and venous sinuses {blue).
1. Dura mater: Dura mater (L. tough mother) is the outermost, thick connective tissue layer. In the skull, the dura has two layers, a periosteal layer that is attached directly to the calvaria and cranial base and a meningeal layer that forms dural inholdings and is continuous with the dura surrounding the spinal cord. These layers are fused together, except for where they separate to form dural venous sinuses. A potential epidural (extradural) space exists between the periosteal dura and skull. Dura is pain sensitive, receiving innervation from branches of the trigeminal nerve (CN V) and vagus nerve (CN X). Meningeal arteries provide vascular supply to the dura and travel in the epidural space.
2. Arachnoid mater: Arachnoid mater is the delicate, weblike intermediate layer. It creates a true subarachnoid space, which contains CSF and cerebral vasculature. Collagenous arachnoid trabeculae connect the arachnoid to the underlying pia mater. Under normal conditions, the arachnoid and dura are tightly associated, obliterating the potential subdural space between these two layers. Specializations, called arachnoid granulations, arise from this layer and project into dural venous sinuses, functioning to transfer CSF into the venous system.
3. Pia mater: Pia mater is the innermost meningeal layer and is closely adhered to the surface of the brain. Pia extends into sulci and fissures, separating the brain substance from CSF in the subarachnoid space. Cerebral vessels pierce the pia through the perivascular space to reach the brain parenchyma.
4. Reflections: Reflections of meningeal dura serve as dividing structures throughout different regions of the cranium.
a. Faix cerebri: The cerebral hemispheres are partially separated by a sickle-shaped reflection called the falx cerebri, which sits in the longitudinal fissure and attaches anteriorly to the crista gali (ethmoid).
b. Tentorium cerebelli and tentorial notch: Posteriorly, the occipital lobes and cerebellum are separated by the tentorium cerebelli (tent of the cerebellum). The tentorium attaches anteriorly to the posterior crinoid processes (sphenoid) and along the petrous ridges of the temporal bones and is interrupted in the midline by the tentorial notch, which allows for the brainstem to enter the posterior cranial fossa and exit the skull. Posteriorly, the tentorium attaches to the parietal and occipital bones.
c. Faix cerebelli: A small falx cerebelli partially separates the cerebellar hemispheres in the posterior cranial fossa.
d. Diaphragma sellae: The diaphragma sellae is the smallest dural reflection, spanning the sellae turcica to cover the pituitary gland.
5. Dural venous sinuses: These primarily occur where there is a separation of periosteal and meningeal dura layers. Select sinuses occur between duplications of meningeal dura. These endotheliallined sinuses drain venous blood and reabsorbed CSF directly or indirectly into the internal jugular veins. The primary sinuses are detailed below and shown in Figure 8. Smaller sinuses include the superior and inferior petrosal sinuses, occipital sinus, sphenoparietal sinus, and cavernous sinus.
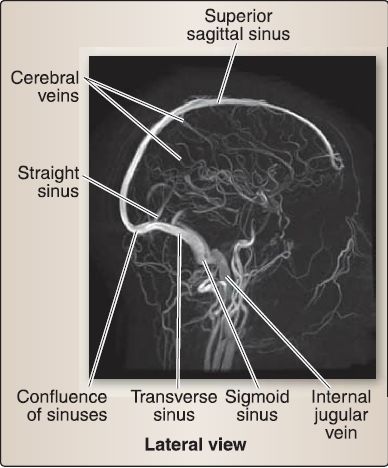
Figure 8: Venography of dural venous sinus system.
a. Superior sagittal sinus: Located in the superior border of the falx cerebri, running from the crista gali to the confluence of sinuses, the superior sagittal sinus is the main site for CSF reabsorption via arachnoid granulations. It receives venous blood from superior cerebral veins and CSF from arachnoid granulations that project directly into the sinus or from small adjacent collections of granulations in lateral venous lacunae.
b. Inferior sagittal sinus: Located in the free border of the falx cerebri, the inferior sagittal sinus terminates in the straight sinus.
c. Straight sinus: Located at the junction of the falx cerebri and tentorium, the straight sinus is formed by the union of the inferior sagittal sinus and the great cerebral vein (of Galen) and terminates at the confluence.
d. Transverse sinus: The bilateral transverse sinus projects laterally from the confluence between a groove in the occipital and parietal bones and the tentorium. It terminates in the sigmoid sinus.
e. Sigmoid sinus: The bilateral S-shaped sigmoid sinus in the posterior cranial fossa exits the skull through the jugular foramen, terminating in the internal jugular vein. It receives venous blood from the superior and inferior petrosal sinuses at its origin and termination, respectively.
f. Confluence of sinuses: The confluence of sinuses is located at the site of the internal occipital protuberance. In general, flow travels from anterior to posterior, where the superior sagittal and straight sinuses drain into the confluence. The confluence also receives the occipital sinus. Venous blood travels laterally from the confluence through the transverse and sigmoid sinuses, before draining into jugular system.
D. Vasculature
Arterial blood supply to the brain arises from multiple sources to allow for adequate flow in the event of peripheral blockage. Specialized dural venous sinuses work not only to drain deoxygenated blood from the brain substance but also to recycle CSF back into the venous system.
1. Arterial supply: Cerebral arterial blood supply arises from branches of the vertebral/basilar arterial and the internal carotid systems (Figs. 9 and 10). Collectively, these two sets of arteries form an arterial circle (circle of Willis), located on the ventral surface of the brain.

Figure 9: Arterial blood supply to the brain. A, Gross specimen with cerebral arterial circle. B, Arterial distribution to cerebrum.

Figure 10 : Arterial circle (of Willis). A, Schematic. B, Angiogram.
a. Vertebral/basilar system: Arising from the subclavian artery, the vertebral artery ascends the cervical region through transverse foramina in cervical vertebra and enters the posterior cranial fossa through the foramen magnum. The pair of vertebral arteries unites near the pontomedullary junction to form the basilar artery. The following branches arise directly from the vertebral and basilar system.
[1] Vertebral arteries: Singular anterior and paired posterior spinal arteries serve the spinal cord, and paired posterior inferior cerebellar arteries serve the cerebellum.
[2] Basilar arteries: Paired anterior inferior cerebellar arteries and paired superior cerebellar arteries serve the cerebellum, multiple pontine arteries serve the pons, and posterior cerebral arteries (PCAs; terminal branches of the basilar artery) serve the occipital lobe.
b. Internal carotid system: Coursing up through the carotid canal, the internal carotid arteries travel through the cavernous sinus and pierce the dura to enter the middle cranial fossa. The following paired branches arise directly from the internal carotid system:
[1] Ophthalmic artery: This artery supplies the eye.
[2] Posterior communicating artery: This artery anastomoses with PCA, thus connecting the internal carotid system to the vertebral/basilar system.
[3] Anterior choroidal artery: This artery supplies portions of the choroid plexus.
[4] Anterior cerebral artery (ACA): The ACA enters the longitudinal fissure, is connected by a short anterior communicating artery, and serves the midline frontal and parietal lobes.
[5] Middle cerebral artery (MCA): The MCA is not considered part of the circle of Willis. It travels laterally between the frontal and temporal lobes and serves lateral temporal,
parietal, and frontal lobes and deep nuclei.
2. Venous supply: Venous blood in the brain is drained by thin, valveless cerebral veins (see Fig. 8). These veins travel with the arteries in the subarachnoid space and must pierce the arachnoid and meningeal dura to drain into the dural venous sinus system. This primarily occurs at the superior sagittal sinus, although a collection of cerebral veins drain deeper structures into the straight sinus by way of the great cerebral vein (of Galen). Transverse and petrosal sinuses also receive cerebral veins.



|
|
|
|
"إنقاص الوزن".. مشروب تقليدي قد يتفوق على حقن "أوزيمبيك"
|
|
|
|
|
|
|
الصين تحقق اختراقا بطائرة مسيرة مزودة بالذكاء الاصطناعي
|
|
|
|
|
|
|
قسم شؤون المعارف ووفد من جامعة البصرة يبحثان سبل تعزيز التعاون المشترك
|
|
|