


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية| The lac Operon Has a Second Layer of Control: Catabolite Repression |
|
|
|
Read More
Date: 6-4-2021
Date: 26-4-2016
Date: 29-11-2015
|
The lac Operon Has a Second Layer of Control: Catabolite Repression
KEY CONCEPTS
- Catabolite repressor protein (CRP) is an activator protein that binds to a target sequence at a promoter.
- A dimer of CRP is activated by a single molecule of cyclic AMP (cAMP).
- cAMP is controlled by the level of glucose in the cell; a low glucose level allows cAMP to be made.
- CRP interacts with the C-terminal domain of the α subunit of RNA polymerase to activate it.
The E. coli lac operon is negative inducible. Transcription is turned on by the presence of lactose by removing the lac repressor. This operon, however, is also under a second layer of control and cannot be turned on by lactose if the bacterium has a sufficient supply of glucose. The rationale for this is that glucose is a better energy source than lactose, so there is no need to turn on the operon if there is glucose available. This system is part of a global network called catabolite repression that affects about 20 operons in E. coli. Catabolite repression is exerted through a second messenger called cyclic AMP (cAMP) and the positive regulator protein called the catabolite repressor protein (CRP) (CRP can also stand for cAMP receptor protein and is also called catabolite activator protein, or CAP). The lac operon is therefore under dual control.
Thus far we have dealt with the promoter as a DNA sequence that is competent to bind RNA polymerase, which then initiates transcription. Some promoters, though, do not allow RNA polymerase to initiate transcription without assistance from an ancillary protein. Such proteins are positive regulators, because their presence is necessary to switch on the transcription unit.
Typically, the activator overcomes a deficiency in the promoter—for example, a poor consensus sequence at −35 or −10, or both. One of the most widely acting activators is CRP. This protein is a positive regulator whose presence is necessary to initiate transcription at dependent promoters. CRP is active only when bound to cAMP, which behaves as a classic small-molecule inducer for positive control (see FIGURE 1).
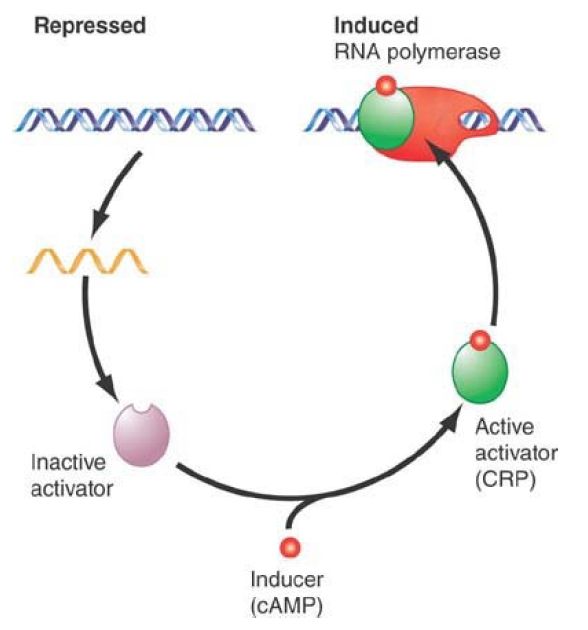
FIGURE 1. A small-molecule inducer, cAMP, converts an activator protein, CRP, to a form that binds the promoter and assists RNA polymerase in initiating transcription.
cAMP is synthesized by the enzyme adenylate cyclase. The reaction uses ATP as substrate and introduces an internal 3′−5′ link via a phosphodiester bond, which generates the structure drawn in FIGURE 2. Adenylate cyclase activity is repressed by high glucose, as shown in FIGURE 3. Thus, the level of cAMP is inversely related to the level of glucose. Only with low levels of glucose is the enzyme active and able to synthesize cAMP. In turn, cAMP binding is required for CRP to bind DNA and activate transcription. Thus, transcription activation by CRP only occurs when cellular glucose levels are low.

FIGURE 2 Cyclic AMP has a single phosphate group connected to both the 3′ and 5′ positions of the sugar ring.

FIGURE 3 By reducing the level of cyclic AMP, glucose inhibits the transcription of operons that require CRP activity.
CRP is a dimer of two identical subunits of 22.5 kD, which can be allosterically activated by a single molecule of cAMP. A CRP monomer contains a DNA-binding region and a transcriptionactivating region. cAMP binding alters the structure of CRP to change the DNA-binding domain from one that binds all DNA weakly to strong, sequence-specific DNA binding. A CRP dimer binds to a site of about 22 bp at a responsive promoter. The binding sites include variations of the 5-bp consensus sequence shown in FIGURE 4 . Mutations preventing CRP action usually are located within the well conserved pentamer, which appears to be the essential element in recognition. CRP binds most strongly to sites that contain two (inverted) versions of the pentamer, because this enables both subunits of the dimer to bind to the DNA.
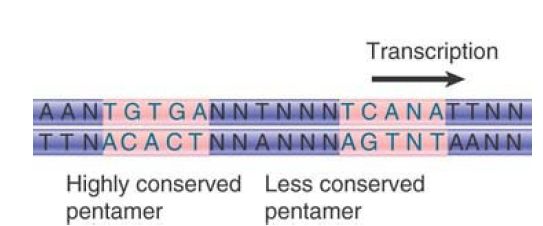
FIGURE 4The consensus sequence for CRP contains the well-conserved pentamer TGTGA and (sometimes) an inversion of this sequence (TCANA).
CRP introduces a large bend when it binds DNA. In the lac promoter, this point lies at the center of dyad symmetry. The bend is quite severe, greater than 90°, as illustrated in FIGURE 5 . Therefore, a dramatic change occurs in the organization of the DNA double helix when CRP protein binds. The mechanism of bending is to introduce a sharp kink within the TGTGA consensus sequence.
When there are inverted repeats of the consensus, the two kinks in each copy present in a palindrome cause the overall 90° bend. It is possible that the bend has some direct effect upon transcription, but it could be the case that it is needed simply to allow CRP to contact RNA polymerase at the promoter.
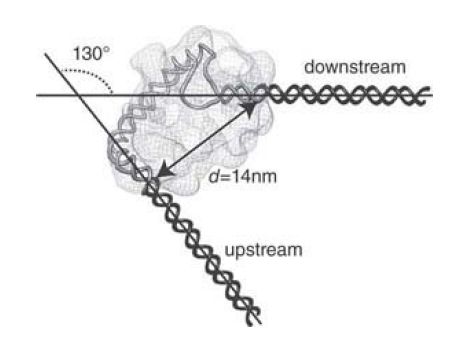
FIGURE 5.CRP bends DNA more than 90° around the center of symmetry. Class I CAP-RNAP-promoter complex electron microscopy (EM) reconstruction and fitted model: inferred path of DNA.
Reproduced from H. P. Hudson, et al., Proc. Natl. Acad. Sci. USA 47 (2009): 19830–19835.
The action of CRP has the curious feature that its binding sites lie at different locations relative to the start point in the various operons that it regulates. The TGTGA pentamer may lie in either orientation. The three examples shown in FIGURE 6 encompass the range of locations:
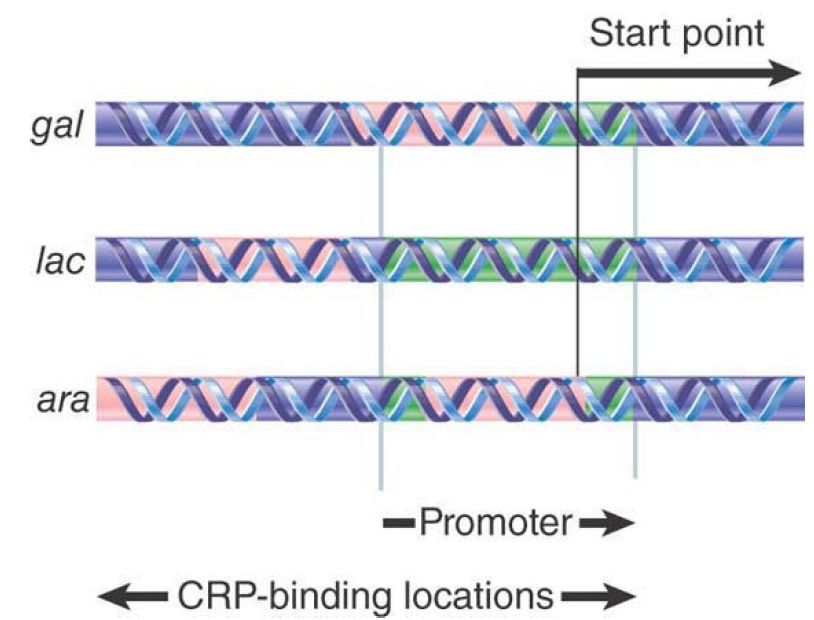
FIGURE 6. The CRP protein can bind at different sites relative to RNA polymerase.
The CRP-binding site is adjacent to the promoter, as in the lac operon, in which the region of DNA protected by CRP is centered on −61. It is possible that two dimers of CRP are bound. The binding pattern is consistent with the presence of CRP largely on one face of DNA, which is the same face that is bound by RNA polymerase. This location would place the two proteins just about in reach of each other.
Sometimes the CRP-binding site lies within the promoter, as in the gal locus, where the CRP-binding site is centered on −41. It is likely that only a single CRP dimer is bound, probably in quite intimate contact with RNA polymerase, because the CRPbinding site extends well into the region generally protected by the RNA polymerase.
In other operons, the CRP-binding site lies well upstream of the promoter. In the ara region, the binding site for a single CRP is the farthest from the start point, centered at −92.
Dependence on CRP is related to the intrinsic efficiency of the promoter. No CRP-dependent promoter has a good −35 sequence, and some also lack good −10 sequences. In fact, it can could be argued that effective control by CRP would be difficult if the promoter had effective −35 and −10 regions that interacted independently with RNA polymerase.
In principle, CRP might activate transcription in one of two ways: It could interact directly with RNA polymerase, or it could act upon DNA to change its structure in some way that assists RNA polymerase to bind. In fact, CRP has effects upon both RNA polymerase and DNA.
Binding sites for CRP at most promoters resemble either lac (centered at −61) or gal (centered at −41 bp). The basic difference between them is that in the first type (called class I) the CRPbinding site is entirely upstream of the promoter, whereas in the second type (called class II) the CRP-binding site overlaps the binding site for RNA polymerase. (The interactions at the ara promoter may be different.)
In both types of promoter, the CRP binding site is centered an integral number of turns of the double helix from the start point. This suggests that CRP is bound to the same face of DNA as RNA polymerase. The nature of the interaction between CRP and RNA polymerase is, however, different at the two types of promoter.
When the α subunit of RNA polymerase has a deletion in the Cterminal end, transcription appears normal except for the loss of ability to be activated by CRP. CRP has an “activating region” that is required for activating both types of its promoters. This activating region, which consists of an exposed loop of approximately 10 amino acids, is a small patch that interacts directly with only one of the two α subunits of RNA polymerase to stimulate the enzyme. At class I promoters, this interaction is sufficient. At class II promoters, a different set of interactions occurs between CRP and the RNA polymerase.
Experiments using CRP dimers in which only one of the subunits has a functional transcription-activating region show that when CRP is bound at the lac promoter only the activating region of the subunit nearer the start point is required, presumably because it touches RNA polymerase. This offers an explanation for the lack of dependence on the orientation of the binding site: The dimeric structure of CRP ensures that one of the subunits is available to contact RNA polymerase, no matter which subunit binds to DNA and in which orientation.
The effect upon RNA polymerase binding depends on the relative locations of the two proteins. At class I promoters, where CRP binds adjacent to the promoter, it increases the rate of initial binding to form a closed complex. At class II promoters, where CRP binds within the promoter, it increases the rate of transition from the closed to open complex.



|
|
|
|
التوتر والسرطان.. علماء يحذرون من "صلة خطيرة"
|
|
|
|
|
|
|
مرآة السيارة: مدى دقة عكسها للصورة الصحيحة
|
|
|
|
|
|
|
نحو شراكة وطنية متكاملة.. الأمين العام للعتبة الحسينية يبحث مع وكيل وزارة الخارجية آفاق التعاون المؤسسي
|
|
|