


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 5-5-2016
Date: 15-5-2016
Date: 23-2-2021
|
Double-Strand Breaks Initiate Recombination
KEY CONCEPTS
- The double-strand break repair (DSBR) model of recombination is initiated by making a double-strand break in one (recipient) DNA duplex and is relevant for meiotic and mitotic homologous recombination.
- Exonuclease action generates 3′–single-stranded ends that invade the other (donor) duplex.
- When a single strand from one duplex displaces its counterpart in the other duplex, it creates a branched structure called a D-loop.
- Strand exchange generates a stretch of heteroduplex DNA consisting of one strand from each parent.
- New DNA synthesis replaces the material that has been degraded.
- Capture of the second double-strand break end by annealing generates a recombinant joint molecule in which the two DNA duplexes are connected by heteroduplex DNA and two Holliday junctions.
- The joint molecule is resolved into two separate duplex molecules by nicking two of the connecting strands.
- Whether recombinants are formed depends on whether the strands involved in the original exchange or the other pair of strands is nicked during resolution.
Genetic exchange is initiated by a double-strand break (DSB). The double-strand break repair (DSBR) model is illustrated in FIGURE 1. Recombination is initiated by an endonuclease that cleaves one of the partner DNA duplexes, the “recipient.” In meiosis this is performed by the Spo11 protein, which is related to DNA topoisomerases (FIGURE 2). DNA topoisomerases are enzymes that catalyze changes in the topology of DNA by transiently breaking one or both strands of DNA, passing theunbro ken strand(s) through the gap, and then resealing the gap.
The ends that are generated by the break are never free, but instead are manipulated exclusively within the confines of the enzyme—in fact, they are covalently linked to the enzyme. Spo11 undergoes a similar covalent attachment when it forms DSBs during meiosis.
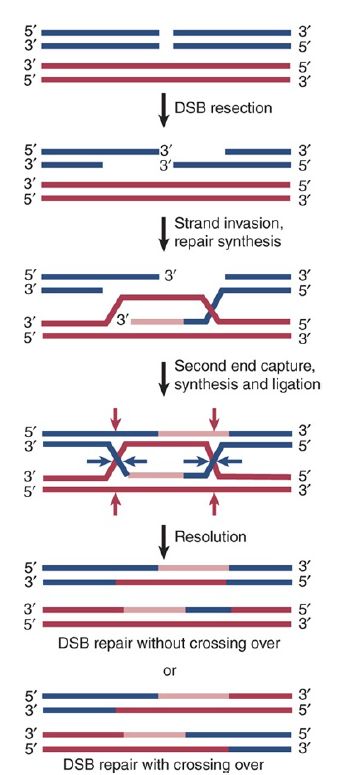
FIGURE 13.4 The double-strand break repair (DSBR) model of homologous recombination. Recombination is initiated by a doublestrand break. Following nuclease degradation of the ends, called DNA resection, single-strand tails with 3′–OH ends are formed. Strand invasion by one end into homologous sequences forms a Dloop. Extension of the 3′–OH end by DNA synthesis enlarges the Dloop. Once the displaced loop can pair with the other side of the break, the second double-strand break end is captured. DNA synthesis to complete the break repair, followed by ligation, results in the formation of two Holliday junctions. Resolution at the blue arrowheads results in a noncrossover product. Resolution of one Holliday junction at the blue arrowheads and the other Holliday junction at the red arrowheads results in a crossover product.
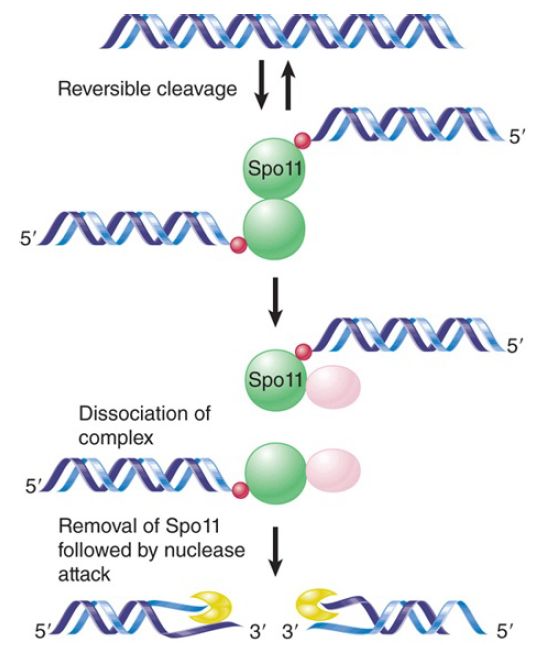
FIGURE 2. Spo11 is covalently joined to the 5′ ends of doublestrand breaks.
In mitotic cells DSBs form spontaneously as a result of DNA damage or through the action of specific processes that are programmed to form breaks, such as V(D)J recombination or mating-type switching in yeast. Exonuclease(s), which can work in concert with a DNA helicase, degrade one strand on either side of the break, generating 3′–single-stranded termini; this process is known as 5′-end resection. In earlier models, this included the formation of a significant gap at the site of the DSB, but more recent data suggest that large gaps are not usually present in vivo.
One of the free 3′ ends then invades a homologous region in the other (“donor”) duplex. This is called single-strand invasion. The formation of heteroduplex DNA generates a D-loop (displacement loop), in which one strand of the donor duplex is displaced. The point at which an individual strand of DNA crosses from one duplex to the other is called the recombinant joint. An important feature of a recombinant joint is its ability to move along the duplex. Such mobility is called branch migration. The D-loop is extended by repair DNA synthesis, using the free 3′ end as a primer to generate double-stranded DNA. FIGURE 3 illustrates the migration of a single strand in a duplex. The branching point can migrate in either direction as one strand is displaced by the other.
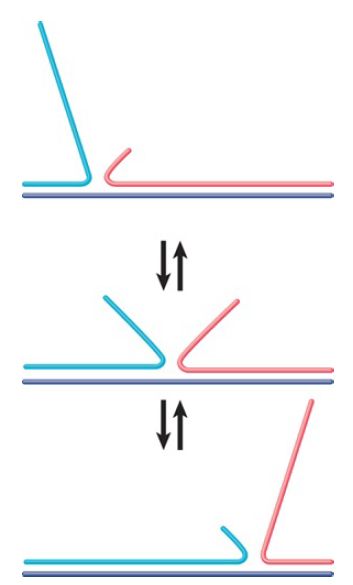
FIGURE 3. Branch migration can occur in either direction when an unpaired single strand displaces a paired strand.
Branch migration is important for both theoretical and practical reasons. As a matter of principle, it confers a dynamic property on recombining structures. As a practical feature, its existence means that the point of branching cannot be established by examining a molecule in vitro (because the branch may have migrated since the molecule was isolated).
Branch migration can allow the point of crossover in the recombination intermediate to move in either direction. The rate of branch migration is uncertain, but, as seen in vitro, it is probably
inadequate to support the formation of extensive regions of heteroduplex DNA in natural conditions. Any extensive branch migration in vivo must therefore be catalyzed by a recombination enzyme.
The second resected single strand subsequently anneals to the donor, forming a second single-end invasion (SEI) and converting the D-loop into two crossed strands or recombinant joints called Holliday junctions. Overall, the resected region has been repaired by two individual rounds of single-strand DNA synthesis. The joints must be resolved by cutting.
If both joints are resolved in the same way, the original noncrossover molecules will be released, each with a region of altered genetic information that is a footprint of the exchange event. If the two joints are resolved in opposite ways, a genetic crossover is produced.
The involvement of DSBs at first seems surprising. Once a break has been made right across a DNA molecule, there is no going back. In the DSBR model, the initial cleavage is immediately
followed by loss of information. Any error in retrieving the information could be fatal. However, the very ability to retrieve lost information by resynthesizing it from another duplex provides a major safety net for the cell.
The joint molecule formed by strand exchange must be resolved into two separate duplex molecules. Resolution requires a further pair of nicks. We can most easily visualize the outcome by viewing the joint molecule in one plane as a Holliday junction. This is illustrated in the bottom half of Figure 1, which represents the resolution reaction. The outcome of the reaction depends on which pair of strands is nicked.
If the nicks are made in the pair of strands that was not originally nicked (the pair that did not initiate the strand exchange), all four of the original strands have been nicked. This releases crossover recombinant DNA molecules. The duplex of one DNA parent is covalently linked to the duplex of the other DNA parent via a stretch of heteroduplex DNA.
If the same two strands involved in the original nicking are nicked again, the other two strands remain intact. The nicking releases the original parental duplexes, which remain intact, with the exception that each has a residuum of the event in the form of a length of heteroduplex DNA. These are noncrossover products that nonetheless contain sequence from the donor DNA duplex, and as such are considered recombinant. Although this descriptionsuggests that the outcome is random, newer evidence suggests that numerous factors influence crossover versus noncrossover outcomes, and the distinction is established as early as the stage of D-loop formation.
What is the minimum length of the region required to establish the connection between the recombining duplexes? Experiments in which short homologous sequences carried by plasmids or phages are introduced into bacteria suggest that the rate of recombination is substantially reduced if the homologous region is less than 75 bp.
This distance is appreciably longer than the 10 bp or so required for association between complementary single-stranded regions, which suggests that recombination imposes demands beyond annealing of complements as such.



|
|
|
|
لخفض ضغط الدم.. دراسة تحدد "تمارين مهمة"
|
|
|
|
|
|
|
طال انتظارها.. ميزة جديدة من "واتساب" تعزز الخصوصية
|
|
|
|
|
|
|
عوائل الشهداء: العتبة العباسية المقدسة سبّاقة في استذكار شهداء العراق عبر فعالياتها وأنشطتها المختلفة
|
|
|