


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 14-3-2021
Date: 10-12-2015
Date: 19-5-2016
|
Nucleic Acid Detection
KEY CONCEPT
- Hybridization of a labeled nucleic acid to complementary sequences can identify specific nucleic acids.
There are a number of different ways to detect DNA and RNA. The classical method relies on the ability of nucleic acids to absorb light at 260 nanometers. The amount of light absorbed is proportional to the amount of nucleic acid present. There is a slight difference in the amount of absorption by single-stranded versus doublestranded nucleic acids, but not DNA versus RNA. Protein contamination can affect the outcome, but because proteins absorb maximally at 280 nm, tables have been published of 260/280 ratios that allow quantitation of the amount of nucleic acid present.
DNA and RNA can be nonspecifically stained with ethidium bromide (EtBr) to make visualization more sensitive. EtBr is an organic tricyclic compound that binds strongly to double-stranded DNA (and RNA) by intercalating into the double helix between the stacked base pairs. It binds to DNA, thus is a strong mutagen and care must be taken when using it. EtBr fluoresces when exposed to ultraviolet (UV) light, which increases the sensitivity. SYBR green is a safer alternate DNA stain.
We now focus on the detection of specific sequences of nucleic acids. The ability to identify a specific sequence relies on hybridization of a probe with a known sequence to a target. The probe can detect and bind to a sequence to which it is complementary. The percentage of match does not need to be perfect, but as the match percentage decreases, the stability of the nucleic acid hybrid decreases. G-C base pairs are more stable than A-T base pairs so that base composition (usually referred to as % G-C) is an important variable. The second set of variables that affects hybrid stability is extrinsic; it includes the buffer conditions (concentration and composition) and the temperature at which hybridization occurs. This is called the stringency, under which the hybridization is carried out.
The probe functions as a single-stranded molecule (if it is double stranded, it must be melted). The target can be single stranded or double stranded. If the target is double stranded, it also must be melted to single strands to begin the hybridization process. The reaction can take place in solution (e.g., during sequencing or PCR), or it can be performed when the target has been
bound to a membrane support such as a nitrocellulose filter . The target can be DNA (called a Southern blot) or RNA (called a Northern blot); the probe is usually DNA.
For this exercise, let’s use a Southern blot from an experiment in which we have restricted a large DNA fragment into smaller fragments and subcloned the individual fragments . Starting with the clones on the plate from Figure 2.5, we can isolate plasmid DNA from each white clone and restrict the DNA with the same restriction enzymes used to clone the fragments. The DNA fragments will be separated on an agarose gel and blotted onto nitrocellulose .
To increase the sensitivity from the optical range, the probe must be labeled. Begin with radiolabeling and then describe alternate labeling without radioactivity. For most reactions, 32P is used, but 33P (with a longer half-life but less penetrating ability) and H (for special purposes described later) are also used. Probes can be radiolabeled in several different ways. One is end labeling, in which a strand of DNA (that has no 5′ phosphate) is labeled by using a kinase and 32P. Alternatively, a probe can be generated by nick translation or random priming with 32P using the Klenow DNA polymerase fragment and labeled nucleotides or during a PCR reaction .
In performing nucleic acid hybridization studies, standard procedures are typically used that allow hybridization over a large range of G-C content. Hybridization experiments are performed in a standardized buffer called standard sodium citrate (SSC), which is usually prepared as a 20× concentrated stock solution. Hybridization is typically carried out within a standard temperature range of 45°C to 65°C, depending upon the required stringency.
The actual hybridization between a labeled probe and a target DNA bound to a membrane usually takes place in a closed (or sealed) container in a buffer that contains a set of molecules to reduce background hybridization of the probe to the filter. Hybridization experiments typically are performed overnight to ensure maximum probe-to-target hybridization. The hybridization reaction is stochastic and depends upon the abundance of each different sequence. The more copies of a sequence, the greater the chance of a given probe molecule encountering its complementary sequence. The next step is to wash the filter to remove all of the probe that is not specifically bound to a complementary sequence of nucleic acid. Depending on the type of experiment, the stringency of the wash is usually set quite high to avoid spurious results. Higher stringency conditions include higher temperature (closer to the
melting temperature of the probe) and lower concentration of cations. (Lower salt concentrations result in less shielding of the negative phosphate groups of the DNA backbone, which in turn inhibits strand annealing.) In some experiments, however, where one is looking specifically for hybridization to targets with a lower percentage of match (e.g., finding a copy of species X DNA using a probe from species Y), hybridization would be performed at lower
stringency.
The last step is the identification of which target DNA band on the gel (and thus the filter) has been bound by the radiolabeled probe. The washed nitrocellulose filter is subjected to autoradiography. The dried filter will be placed against a sheet of x-ray film. To amplify the radioactive signal, intensifying screens can be used.
These are special screens placed on either side of the filter/film pair that act to bounce the radiation back through the film. Alternatively, a phosphorimaging screen (a solid-state liquid scintillation device) can be used. This is more sensitive and faster than X-ray film, but results in somewhat lower resolution. The length of time for autoradiography is empirical. An estimate of the total radioactivity can be made with a handheld radiation monitor.
Sample results are shown in FIGURE 1. One band on the filter has blackened the X-ray film. The film can be aligned to the filter to determine which band corresponds to the probe.

FIGURE .1 A cartoon of an autoradiogram of a gel prepared from the colonies.
The gel was blotted onto nitrocellulose and probed with a radioactive gene fragment. Lane 1 contains a set of standard DNA size markers. Lane 2 is the original vector cleaved with EcoR1. Lanes 3 to 6 each contain plasmid DNA from one of the white clones that was restricted with EcoR1. A cartoon of the photograph of the gel is on the left; the radioactive bands are marked with an asterisk.
Using a simple modification of the autoradiography procedure called in situ hybridization allows one to peer into a cell and determine the location, at a microscopic level, of specific nucleic acid sequences. We simply modify a few steps in the process to perform the hybridization between our probe, usually labeled with 3H, and complementary nucleic acids in an intact cell or tissue. The goal is to determine exactly where the target is located. The cell or
tissue slice is mounted on a microscope slide. Following hybridization, a photographic emulsion instead of film is applied to the slide, covering it. The emulsion, when developed, is transparent to visible light so that it is possible to see the exact location in the cell where the grains in the emulsion blackened by the radioactivity are located. Development time can be weeks to months because 3H has less energetic radiation and its longer half-life results in lower activity.
There are nonradioactive alternatives to the procedures described here that use either colorimetric or fluorescence labeling. A digoxygenin-labeled probe is a commonly used colorimetric procedure. The probe bound to target is localized with an antidigoxygenin antibody coupled to alkaline phosphatase to develop color. The advantage is the time required to see the results. It is typically a single day, but sensitivity is usually less than with radioactivity. Fluorescence in situ hybridization (FISH) is another very common nonradioactive procedure that uses a fluorescently labeled probe. This method is illustrated in FIGURE 2. Multiple fluorophores in different colors are available—about a dozen now—
but ratios of different probe color combinations can be used to create additional colors.
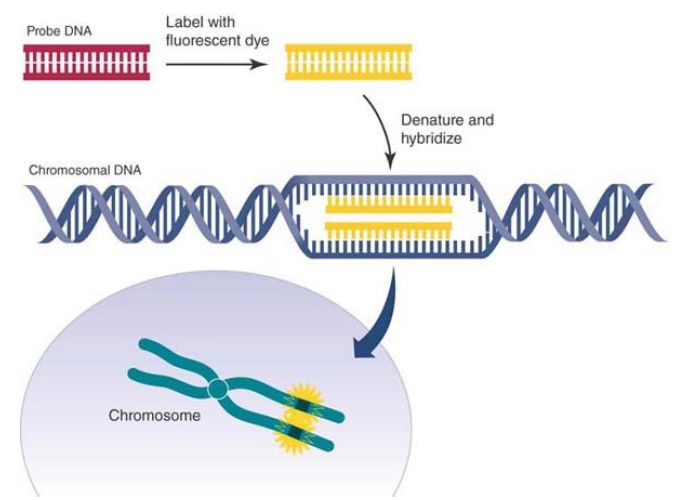
FIGURE 2. Fluorescence in situ hybridization (FISH).
Data from an illustration by Darryl Leja, National Human Genome Research Institute
(www.genome.gov).
These procedures are more picturesque but less quantitative than traditional scintillation counting. At best, they can be called semiquantitative. It is possible to use an optical scanner to
quantitate the amount of signal produced on film, but care must be taken to ensure the time of exposure during the experiment is within a linear range.



|
|
|
|
التوتر والسرطان.. علماء يحذرون من "صلة خطيرة"
|
|
|
|
|
|
|
مرآة السيارة: مدى دقة عكسها للصورة الصحيحة
|
|
|
|
|
|
|
نحو شراكة وطنية متكاملة.. الأمين العام للعتبة الحسينية يبحث مع وكيل وزارة الخارجية آفاق التعاون المؤسسي
|
|
|