


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 7-12-2019
Date: 8-12-2019
Date: 3-12-2019
|
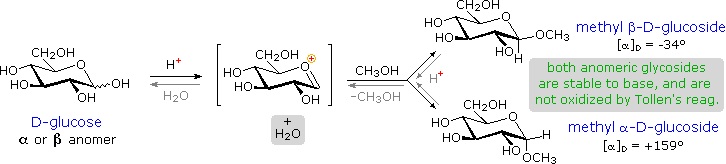
Acetal derivatives formed when a monosaccharide reacts with an alcohol in the presence of an acid catalyst are called glycosides. This reaction is illustrated for glucose and methanol in the diagram below. In naming of glycosides, the "ose" suffix of the sugar name is replaced by "oside", and the alcohol group name is placed first. As is generally true for most acetals, glycoside formation involves the loss of an equivalent of water. The diether product is stable to base and alkaline oxidants such as Tollen's reagent. Since acid-catalyzed aldolization is reversible, glycosides may be hydrolyzed back to their alcohol and sugar components by aqueous acid.
The anomeric methyl glucosides are formed in an equilibrium ratio of 66% alpha to 34% beta. From the structures in the previous diagram, we see that pyranose rings prefer chair conformations in which the largest number of substituents are equatorial. In the case of glucose, the substituents on the beta-anomer are all equatorial, whereas the C-1 substituent in the alpha-anomer changes to axial. Since substituents on cyclohexane rings prefer an equatorial location over axial (methoxycyclohexane is 75% equatorial), the preference for alpha-glycopyranoside formation is unexpected, and is referred to as the anomeric effect.
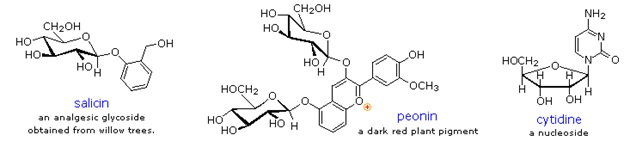
Glycosides abound in biological systems. By attaching a sugar moiety to a lipid or benzenoid structure, the solubility and other properties of the compound may be changed substantially. Because of the important modifying influence of such derivatization, numerous enzyme systems, known as glycosidases, have evolved for the attachment and removal of sugars from alcohols, phenols and amines. Chemists refer to the sugar component of natural glycosides as the glycon and the alcohol component as the aglycon.
Two examples of naturally occurring glycosides and one example of an amino derivative are displayed above. Salicin, one of the oldest herbal remedies known, was the model for the synthetic analgesic aspirin. A large class of hydroxylated, aromatic oxonium cations called anthocyanins provide the red, purple and blue colors of many flowers, fruits and some vegetables. Peonin is one example of this class of natural pigments, which exhibit a pronounced pH color dependence. The oxonium moiety is only stable in acidic environments, and the color changes or disappears when base is added. The complex changes that occur when wine is fermented and stored are in part associated with glycosides of anthocyanins. Finally, amino derivatives of ribose, such as cytidine play important roles in biological phosphorylating agents, coenzymes and information transport and storage materials.



|
|
|
|
التوتر والسرطان.. علماء يحذرون من "صلة خطيرة"
|
|
|
|
|
|
|
مرآة السيارة: مدى دقة عكسها للصورة الصحيحة
|
|
|
|
|
|
|
نحو شراكة وطنية متكاملة.. الأمين العام للعتبة الحسينية يبحث مع وكيل وزارة الخارجية آفاق التعاون المؤسسي
|
|
|