


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 6-9-2019
Date: 28-10-2019
Date: 1-12-2019
|
Oxygen has two lone pairs of electrons hanging around. These electrons make the oxygen more electronegative than carbon. The carbon is then partially postive (electrophillic) and the oxygen partially negative (nucleophillic). The polarizability is denoted by a lowercase delta and a positive or negative superscript depending. For example, carbon would have d+ and oxygen delta^(-). The polarization of carbonyl groups also effects the boiling point of aldehydes and ketones to be higher than those of hydrocarbons in the same amount. The larger the carbonyl compound the less soluble it is in water. If the compound exceeds six carbons it then becomes insoluble.
*For more information about carbonyl solubility, look in the "outside links" section
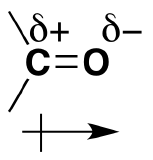
*Amides are the most stable of the carbonyl couplings due to the high-resonance stabilization between nitrogen-carbon and carbon-oxygen.



|
|
|
|
التوتر والسرطان.. علماء يحذرون من "صلة خطيرة"
|
|
|
|
|
|
|
مرآة السيارة: مدى دقة عكسها للصورة الصحيحة
|
|
|
|
|
|
|
نحو شراكة وطنية متكاملة.. الأمين العام للعتبة الحسينية يبحث مع وكيل وزارة الخارجية آفاق التعاون المؤسسي
|
|
|